Shutterstock.com
Almost one-third of people (32%) responding to an Ipsos poll said they were opposed to offering weight-loss injections on the NHS to people with obesity.
The results of the survey of 1,076 people, carried out between 15 October and 28 October 2024, were published soon after NHS England published proposals in October 2024 for a phased launch of the obesity injection tirzepatide (Mounjaro; Eli Lilly), which it estimates could be rolled out to treat 1.6 million people by 2036.
However, the survey results also showed that 37% of British adults surveyed believe that people with obesity should be offered weight-loss injections on the NHS.
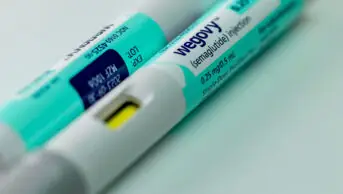
Weight-loss injections are glucagon-like peptide-1 receptor agonists (GLP-1 RA), which come as pre-filled pens, with Wegovy (semaglutide; Novo Nordisk) available on the NHS for obesity through specialist weight management clinics.
The current guidance — ‘Accessing Wegovy for weight loss: everything you need to know‘ — published in September 2023, says that Ozempic (semaglutide; Novo Nordisk) should “only be prescribed for the treatment of type 2 diabetes [mellitus] to protect supply for diabetes patients” and should not be prescribed for weight loss.
According to the survey, around one-quarter (24%) of respondents said they would consider using the injections in future if they were provided by the NHS, with the figure rising to 49% among those who self-reported as obese.
However, 7% of respondents said they would consider paying privately for the injections, with this figure rising to 17% among those who considered themselves obese.
Paul Wright, consultant cardiovascular pharmacist at Barts Health NHS Trust, said there was a need for caution when interpreting the data.
“The self-reported rates of overweight and obesity in the sample was 16% and 7%, respectively, whereas NHS Digital suggests much higher rates of 64% and 29%, respectively,” he said.
“This is important as the poll also suggested more support to give weight-loss injections for those patients who were self-reported as being obese.”
“We are seeing many benefits to injectable weight loss drugs that are seen with sustained long-term weight loss. As expected with weight loss, you see improvements in many cardiovascular risk factors, including improvements in insulin sensitivity, lower blood glucose levels, BP improvements and cardiovascular protection,” Wright added.
“In patients who can tolerate these agents, there are many benefits, and allowing access on the NHS will lead to improvements and reductions in many conditions associated with obesity.”
In November 2024, the British Generic Manufacturer’s Association said that the expiry of patent protection on Novo Nordisk’s Saxendra and Victoza (liraglutide) meant that a first wave of generic weight-loss medicines could be made available in the UK.