Shutterstock.com
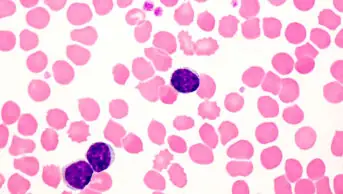
Children and young people in England with a specific form of leukaemia could benefit from personalised cancer treatment within weeks, in the form of chimeric antigen receptor T-cell (CAR-T) therapy, according to Simon Stevens, chief executive at NHS England.
The CAR-T therapy, tisagenlecleucel (Kymriah; Novartis), which was approved by the European Commission on 27 August 2018, is indicated for the treatment of paediatric and young adult patients with B-cell acute lymphoblastic leukaemia that is refractory or in second or later relapse. It is also indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy.
CAR-T therapies are a new generation of personalised cancer immunotherapies that involve engineering a patient’s own immune cells to target their cancer.
Announcing the news at the Health Innovation Expo in Manchester on 5 September 2018, Stevens said that NHS England had agreed the first commercial deal in Europe with Novartis just ten days after the treatment was granted its European marketing authorisation, representing one of the fastest funding approvals in the history of the NHS.
“CAR-T therapy is a true game-changer, and NHS cancer patients are now going to be amongst the first in the world to benefit,” he said.
“This constructive fast-track negotiation also shows how responsible and flexible life sciences companies can succeed — in partnership with the NHS — to make revolutionary treatments available to patients.”
Tisagenlecleucel was one of the first treatments to be supported through the European Medicines Agency’s priority medicines (PRIME) scheme. PRIME, which is voluntary, aims to provide early support for medicines that have the potential to address unmet medical need.