Shutterstock.com
More than 300 take-home naloxone kits have been distributed by community pharmacies in two areas of England over the past year as part of a pilot project, according to figures shared with The Pharmaceutical Journal.
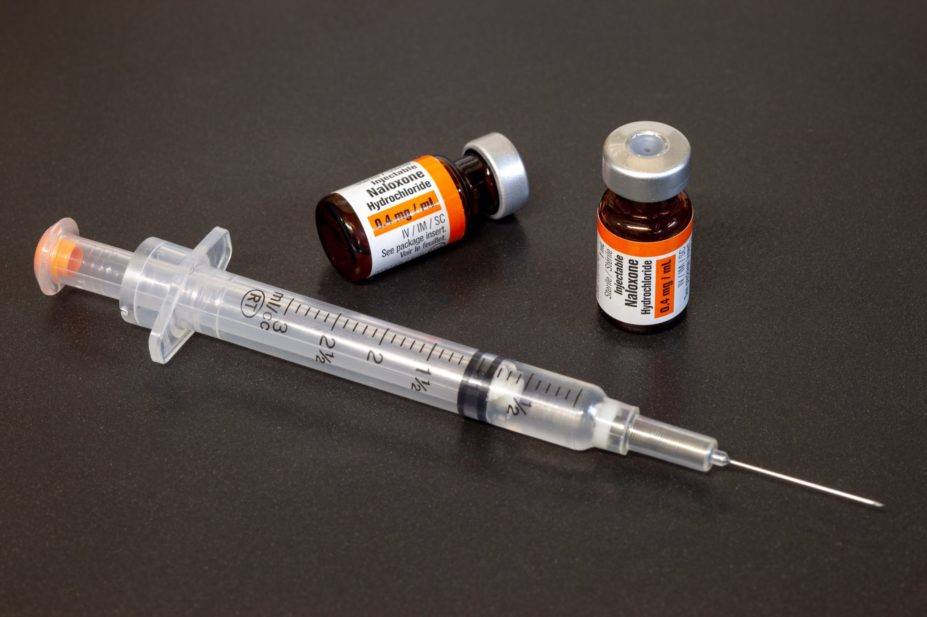
Naloxone is the emergency antidote for overdoses caused by heroin and other opioids, such as methadone, morphine and fentanyl.
A take-home naloxone pilot based in community pharmacies in Somerset and Wakefield, which has been running since May 2020, has reported a supply of more than 330 kits to patients in its first year.
The scheme, set up by charity Turning Point, involves members of the pharmacy team supplying naloxone injections to people either at risk of having, or who might witness someone having, an opioid-related overdose, so that the injection can be administered during an emergency.
Of the kits supplied, most were given in Wakefield, where just 2 pharmacies supplied more than 200 kits. In Somerset, 11 community pharmacies supplied 123 kits in the area.
Supply of the drug can be made by any member of the team in eligible pharmacies once they have completed the mandatory training.
Jenny Scott, a senior lecturer in pharmacy practice at the University of Bath, who works as part of the Turning Point pilot, said she had hoped the numbers of interventions would have been higher but said that the key was to keep naloxone on the community pharmacy’s radar.
In Wales, the ‘Substance Misuse Annual Report‘ for 2019/2020 revealed that 4,833 take-home naloxone kits were supplied through registered sites between April 2019 and March 2020; an increase of 14% from the previous year and the highest number of kits provided in a single financial year.
Just 21 of these kits were supplied by community pharmacies, but a spokesperson for the Welsh government said that it was looking to increase pharmacy provision of the drug.
“Pharmacy distribution of take-home naloxone remains low, but we are working with Community Pharmacy Wales to extend the provision of naloxone within pharmacies that are offering needle and syringe provision,” they said.
In Scotland, a nationwide marketing campaign has been launched by the Scottish government and Scottish Drugs Forum to help raise public awareness of the signs of a drug overdose and the use of naloxone.
TV and radio adverts as well as billboards at transport hubs and shopping centres are aimed to encourage people to go to the StopTheDeaths website to learn how to identify when someone is experiencing an overdose, as well as how to get a naloxone kit and be trained to use it.
Speaking to The Pharmaceutical Journal, Angela Constance, Scottish minister for drug policy, said: “Part of that is about wider engagement with the wider public about how they can equip themselves to save a life, but it has a really important part in tackling stigma.”
In August 2021, the four UK national governments set up a consultation to collect views on expanding the use of naloxone in the UK.
Naloxone is currently a prescription-only medicine regulated by the Human Medicines Regulations 2012. This means that there are controls on who can legally administer, sell and supply naloxone.
Drug treatment services that can currently lawfully supply naloxone without a prescription include drug services provided in primary and secondary care; needle and syringe programmes, including those provided by pharmacies; and pharmacies providing drug treatment, such as opioid substitution treatment.
The consultation will close on the 28 September 2021.