Shutterstock.com
The anticoagulant heparin, designed to treat inherited blood clotting, does not increase the chances of having a baby in women with a history of recurrent miscarriage, a National Institute for Health and Care Research (NIHR)-funded study has revealed.
Researchers looked at the use of low-molecular-weight heparin in treating women with the inherited blood clotting condition thrombophilia.
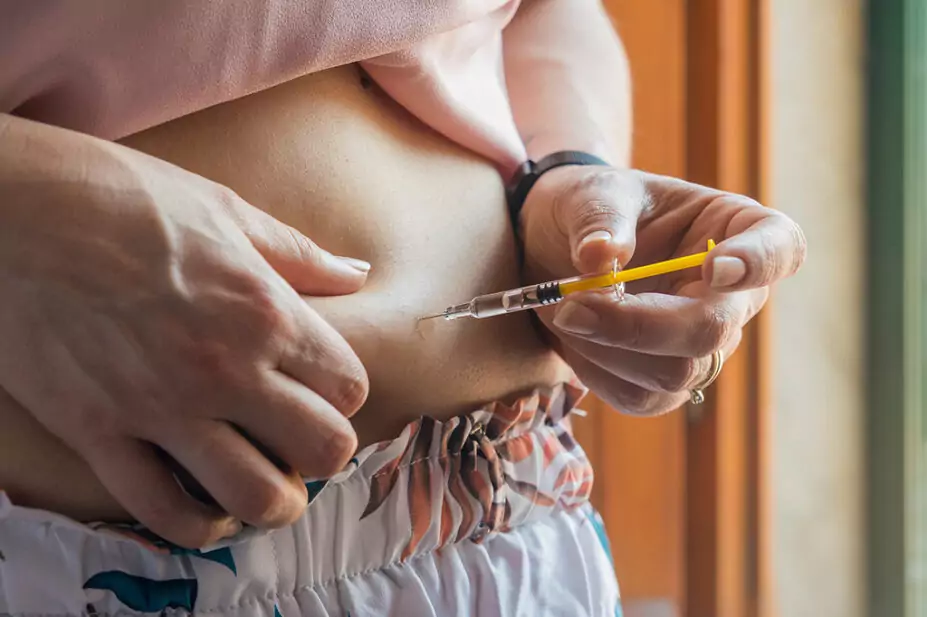
Announcing the study’s findings on 1 June 2023, the NIHR said the NHS could save £20m per year if it stopped screening for inherited thrombophilia during pregnancy and ended the use of heparin for inherited thrombophilia during pregnancy. It added that this would also mean women would no longer have to undergo daily injections as a result, which can lead to bruising.
Findings of the ALIFE2 trial, published in The Lancet on 1 June 2023, were based on 326 women with inherited thrombophilia and recurrent miscarriage from 40 hospitals in the UK, Netherlands, United States, Belgium and Slovenia.
Of those, 164 women were assigned to receive heparin, following a positive pregnancy test, across the course of their pregnancy, while 162 were not offered the medication during the trial.
All women involved in the study received standard obstetrician-led care and were encouraged to take folic acid.
Results revealed that the rate of live births for each group was almost the same, with 116 (72%) of the women taking heparin experiencing live births, compared with 112 (71%) women in the standard care group. The risk of other pregnancy complications (including miscarriage, babies with low birth weight, placental abruption and premature birth or pre-eclampsia) was also almost equal for the two groups, with 39 (24%) women in the heparin group reporting adverse events, compared with 37 (23%) women in the standard care group.
During the trial, 28% of women who took part lost their pregnancies.
Siobhan Quenby, deputy director of Tommy’s National Centre for Miscarriage Research and professor of obstetrics at the University of Warwick, who led the trial, said: “Many women with recurrent miscarriage around the world are tested for inherited thrombophilia and are treated with heparin daily.
“Research now shows that this screening is not needed, the treatment isn’t effective, and it is giving false hope to many by continuing to offer it as a potential preventive treatment.”
Speaking in a personal capacity, research pharmacist Zoë van Zuylen, who is currently on secondment from her role as lead women’s pharmacist at Imperial College Healthcare NHS Trust, said: “This was a well-conducted trial, which will be a welcome addition to the evidence for management of women with recurrent miscarriage.
“It is encouraging to see good-quality trials being undertaken in this underserved area of medicine. However, as good as this trial is, it is important to note that it is just one trial and guidance is based on many factors.”
She added that Royal College of Obstetricians and Gynaecologists guidance on the treatment of recurrent miscarriage was currently being reviewed and updated.