Science Photo Library
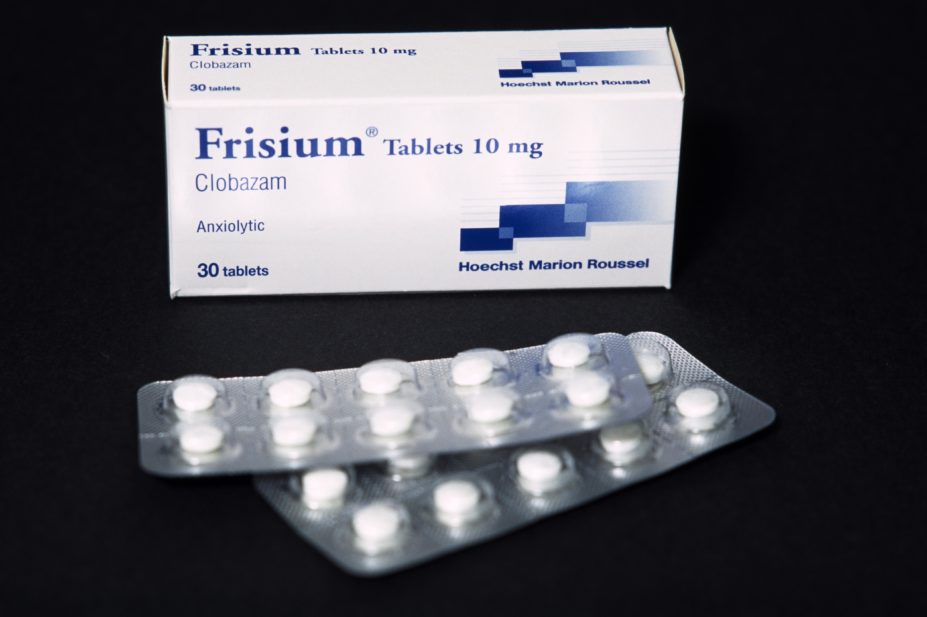
A drug–drug interaction between cannabidiol and anti-epileptic drug clobazam may explain why cannabidiol reduces the frequency of seizures in patients with a severe form of epilepsy, researchers have reported in the British Journal of Clinical Pharmacology (28 October 2019)[1]
.
The research team used clinical trial simulations to investigate the effect of cannabidiol 20mg/kg/day on drop-seizure frequency in patients with Lennox-Gastaut syndrome.
Using population-pharmacokinetic models, the researchers simulated the GWPCARE3 trial — one of the trials that led the US Food and Drug Administration to approve the use of cannabidiol as an anti-epileptic drug.
In the simulation, patients taking 10mg or 20mg clobazam were assumed to have a two- to seven-fold increase in N-desmethylclobazam exposure.
Findings from the simulation suggest that the effect of cannabidiol on the reduction in seizure frequency observed in previous clinical trials may be explained by a six-fold increase in N-desmethylclobazam exposure, which occurs when patients are treated with both 20mg clobazam and cannabidiol.
Geert Jan Groeneveld, chief scientific and medical officer at the Centre for Human Drug Research in Leiden, the Netherlands, said: “The effects of cannabidiol on seizure frequency in Lennox-Gastaut patients could be explained entirely through estimated elevations of blood levels of clobazam, which might mean that cannabidiol in itself may not have any, or at best limited, anti-epileptic effects.”
References
[1] Bergmann K, Broekhuizen K & Groeneveld G. Clinical trial simulations of the interactions between cannabidiol and clobazam and effect on drop-seizure frequency. Br J Clin Pharmacol 2019. doi: 10.1111/bcp.14158


