Shutterstock.com
The European Medicines Agency (EMA) has announced that it is reviewing the diabetes drug canagliflozin after an ongoing clinical trial found it may increase the rate of lower limb amputations.
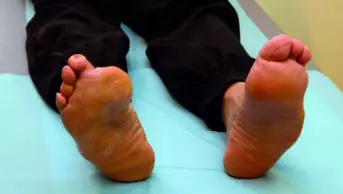
The incidence of amputation in the Canagliflozin Cardiovascular Assessment Study (CANVAS) is currently 7 in 1,000 patient-years among participants treated with canagliflozin 100mg daily and 5 in 1,000 among those receiving the 300mg daily dose. This compares with a rate of 3 in 1,000 patient-years in the placebo group. The incidence of lower limb amputation is primarily related to toe amputation.
The study has enrolled around 4,300 patients who have so far been followed up for an average of 4.5 years.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) has asked for additional information from the drug’s manufacturer to allow it to assess the drug’s association with amputations. It will also look at data on other sodium glucose co-transporter 2 inhibitors.
The EMA says that none of the 12 previous clinical trials involving canagliflozin identified a link with amputations. Another ongoing trial called CANVAS-R, in which patients have so far been followed up for an average of nine months, has shown a non-significant increase in the rate of amputations compared with placebo.
Both trials are designed to assess the effect of canagliflozin on cardiovascular disease and involve patients at high-risk of cardiovascular events. The trials’ independent data monitoring committee has recommended that the trials continue.
Following the review by the PRAC, the Committee for Medicinal Products for Human Use will adopt an opinion on the use of the drug in the EU.