BSIP SA / Alamy Stock Photo
The Medicines and Healthcare products Regulatory Agency (MHRA), which regulates drugs and devices in the UK, has updated its advice concerning the increased risk of lower limb amputation associated with canaglifozin, a drug used to treat type 2 diabetes.
The move follows a recommendation from the European Medicines Agency (EMA), which evaluates medicinal products for use in Europe, that a warning about the possible risks of toe amputation should be added to the prescribing information for canagliflozin and other sodium-glucose co-transporter 2 (SGLT2) inhibitors.
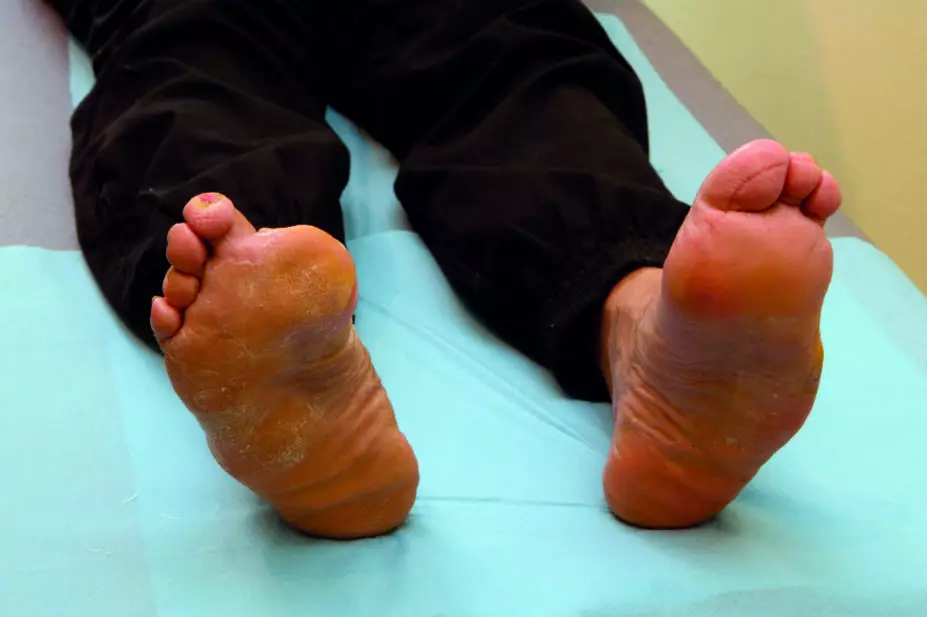
The MHRA confirmed in a drug safety update on 22 March 2017 that the prescribing information for canagliflozin will also list lower limb amputation as an uncommon side effect (occurring in fewer than 10 patients per 1,000)[1]
.
The updates from the MHRA and EMA follow a review of data from two ongoing canagliflozin clinical trials, CANVAS and CANVAS-R, the results of which show that the SGLT2 inhibitor potentially increases the risk of lower limb amputation. According to trial data seen by the MHRA, 6 of the 243 British patients involved in the two trials have had to undergo amputations.
Once approved by the European Commission, the warning about an increased risk of amputation will be added to the summary of product characteristics and package leaflet for canagliflozin, as well as for two other SGLT2 products — dapagliflozin and empagliflozin.
These changes were recommended by the EMA’s Committee for Medicinal Products for Human Use at its meeting on 23 February 2017.
Preliminary data from the CANVAS study show that the incidence of lower limb amputation (mostly affecting the toes) was 7 in 1,000 patient-years with canagliflozin 100mg daily and 5 in 1,000 patient-years with canagliflozin 300mg daily. The amputation rate was 3 in 1,000 patient-years for patients who received a placebo.
In the CANVAS-R study, the incidence of lower limb amputation was 8 in 1,000 patient-years with canagliflozin, compared with 4 in 1,000 patient-years in the placebo group.
However, the EMA points out that the preliminary data are not final because the two studies are ongoing and that “no imbalances were seen in other studies”.
The EMA adds that the trials’ independent data monitoring committee — which has access to all unblinded outcome data and data on side effects — recommended in March 2016 that the two studies should continue and participants be informed of the risk.
The MHRA drug safety update points out that evidence does not show an increased risk for dapagliflozin and empagliflozin, but that the risk may be a class effect.
As such, the MHRA advises healthcare professionals to monitor patients receiving canagliflozin who have risk factors for amputation, such as poor control of diabetes and problems with the heart and blood vessels, and to consider stopping the drug if patients develop foot complications such as infection, skin ulcers, osteomyelitis or gangrene.
It also says patients receiving any SGLT2 inhibitor should be advised about the importance of routine preventive foot care and adequate hydration.
SGLT2 inhibitor-containing medicines that are marketed in the UK include: Invokana (canagliflozin; Janssen), Vokanamet (canagliflozin and metformin; Janssen), Forxiga (dapagliflozin; AstraZeneca), Xigduo (dapagliflozin and metformin; AstraZeneca), Jardiance (empagliflozin; Boehringer Ingelheim), and Synjardy (empagliflozin and metformin; Boehringer Ingelheim).
In a statement, Janssen, the sponsor of the CANVAS studies, says it is taking the amputation risk “very seriously” and has been “proactive in our approach to notifying healthcare professionals and the relevant regulatory bodies around the world about this information”.
The company adds that it followed the independent data monitoring committee’s advice that the CANVAS studies should continue towards their planned endpoint and that patients involved in the trial were informed about the potential risk and were free to withdraw from it at any time.
Janssen adds: “Importantly, the higher incidence seen within the CANVAS programme, which enrolled patients whose diabetes was not well controlled at the beginning of the studies and who also have a history of cardiovascular events or are deemed to be at a high risk for such events, was not observed across the other 12 completed phase III and phase IV clinical trials or in the post-marketing safety monitoring reports.”
The company says it remains confident that canagliflozin is an important treatment option for people with type 2 diabetes.
References
[1] Medicines and Healthcare products Regulatory Agency. Drug safety update. SGLT2 inhibitors: updated advice on increased risk of lower-limb amputation (mainly toes). Volume 10 issue 8, March 2017: 1