Shutterstock.com
The US Food and Drug Administration (FDA), which is responsible for evaluating the safety and efficacy of medicines in the United States, has approved the use of dupilumab (Dupixent; Regeneron Pharmaceuticals) to treat adults with atopic dermatitis.
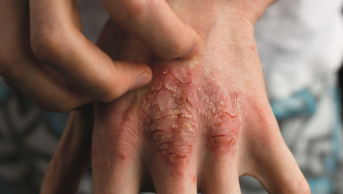
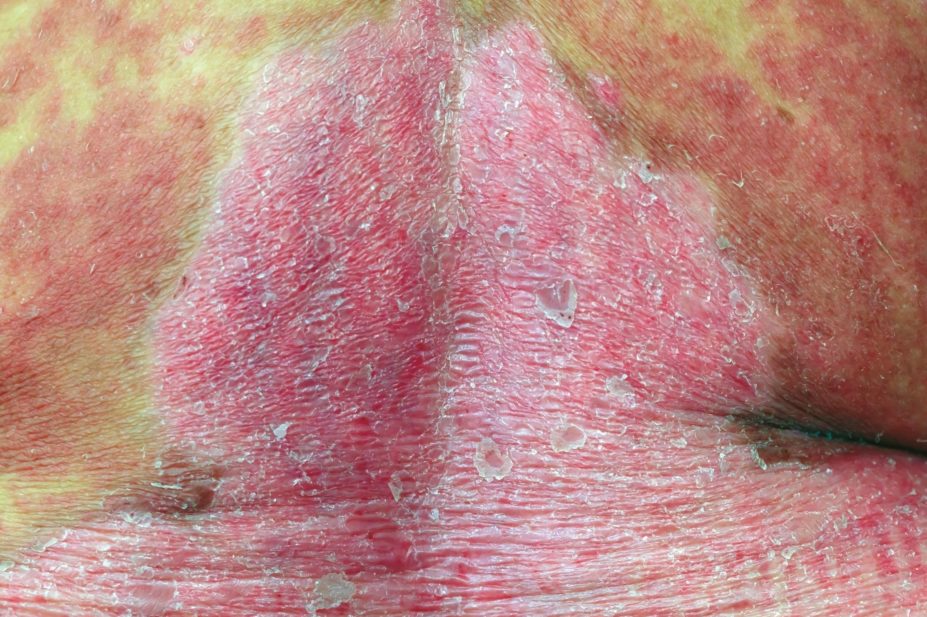
Dupilumab is the first monoclonal antibody to be developed for atopic dermatitis and is anticipated as a revolutionary treatment for patients with severe eczema. It is given as a subcutaneous injection and works by targeting the Th2 pathway to inhibit the inflammatory response that drives atopic dermatitis.
Julie Beitz, director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, says: “The FDA’s approval of Dupixent demonstrates our commitment to approving new and innovative therapies for patients with skin disease.
“Eczema can cause significant skin irritation and discomfort for patients, so it is important to have a variety of treatment options available to patients, including those patients whose disease is not controlled by topical therapies,” she adds.
The safety and efficacy of dupilumab were established in three placebo-controlled clinical trials involving 2,119 adults with moderate-to-severe atopic dermatitis not adequately controlled by topical medication. After 16 weeks of treatment, participants who received dupilumab achieved a greater response compared to controls, and were defined as having ‘clear’ or ‘almost clear’ skin, as well as a reduction in itching.
Side effects associated with the drug include injection site reactions; cold sores in and around the mouth; and redness, swelling and itching around the eyes.
Dupilumab has been under review for marketing authorisation by the European Medicines Agency (EMA) since December 2016.
However, in March 2017, the UK Medicines and Healthcare products Regulatory Agency, which regulates medicines and medical devices in the UK, issued a positive opinion for dupilumab in atopic dermatitis under the ‘Early access to medicines scheme’, meaning that eligible patients will be able to receive the treatment despite it not yet having a marketing authorisation in the EU.