Zephyr / Science Photo Library
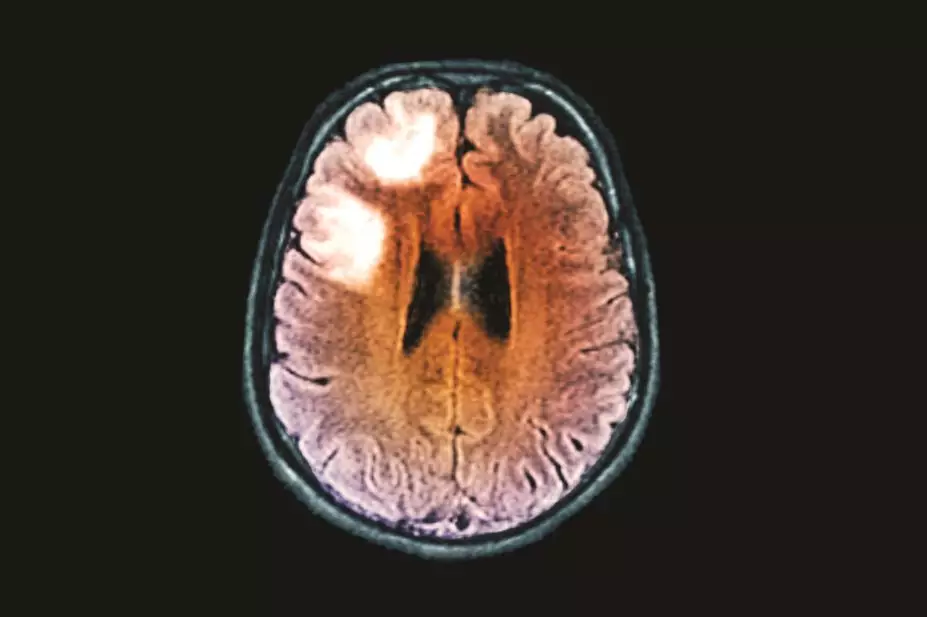
The US Food and Drug Administration (FDA) has issued a new safety warning about cases of a rare brain infection in multiple sclerosis (MS) patients taking fingolimod (Gilenya). It is the first time that cases of the infection — progressive multifocal leukoencephalopathy (PML) — have been reported in patients taking fingolimod who had not been previously treated with an immunosuppressant drug.
Fingolimod, an immunomodulator used to treat relapsing-remitting MS, is approved in the United States and across Europe, including in the UK. It reduces inflammation by altering the autoimmune response held responsible for MS symptoms.
The FDA warning follows one definite and one probable case of PML, a rare but serious viral disease of the brain. Information about these recent cases is now being added to the drug label.
PML is caused by the John Cunningham (JC) virus, which is common, but harmless in most people. It can cause PML in some patients who have impaired immune systems, including those taking immunosuppressant drugs.
The FDA is updating its prescribing information for fingolimod and says that patients taking the drug should contact their health care professionals straight away if they experience symptoms such as new or worsening weakness; increased trouble using their arms or legs; or changes in thinking, eyesight, strength, or balance. Patients should not stop taking fingolimod without first discussing it with their health care professionals, who should stop treatment with the drug and perform a diagnostic evaluation if PML is suspected.
In May 2015, the European Medicines Agency (EMA) recommended that fingolimod’s product information be updated to include a warning about the risk of PML in patients being treated with the drug.