DR P. MARAZZI/SCIENCE PHOTO LIBRARY
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus
A clinical trial to determine the effects of using different COVID-19 vaccines for the first and second dose of a two-dose regimen has been launched in the UK.
The COVID-19 Heterologous Prime Boost study, or ‘Com-Cov’, will also monitor the impact of the different dosing regimens on patients’ immune responses.
Backed by £7m of government funding, the study is being run by the National Immunisation Schedule Evaluation Consortium across eight National Institute for Health Research supported sites — including London, Birmingham and Liverpool — and will involve more than 800 participants.
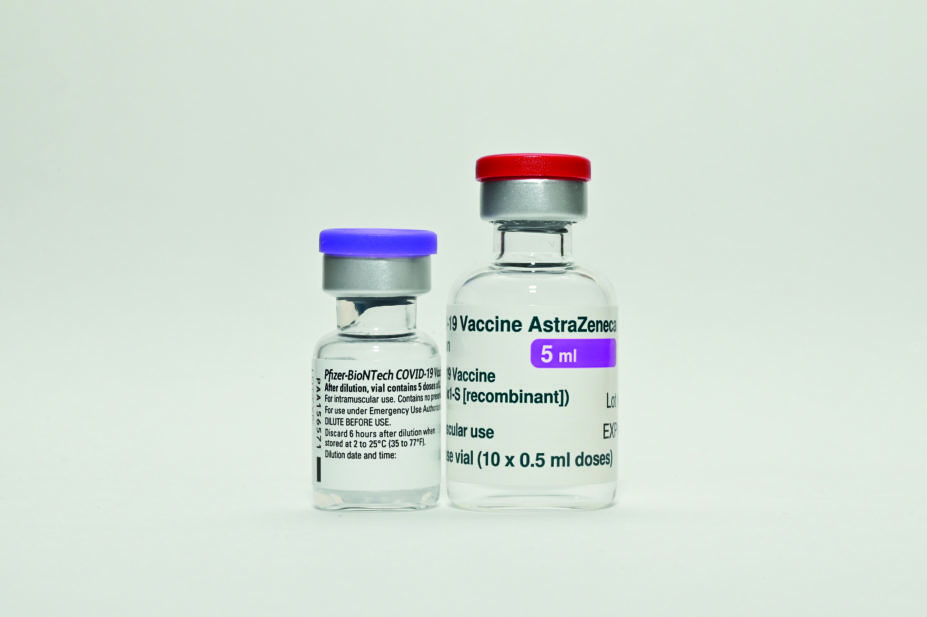
It will initially have eight different arms (see Box), testing eight combinations of the Pfizer/BioNTech and Oxford/AstraZeneca vaccines, but more vaccines may be added at a later stage.
As well as assessing the safety of using different vaccines for each of the doses, the researchers will also gather immunological evidence on different intervals between the first and second dose for a mixed-vaccine regimen, against control groups when the same vaccine is used for both doses. Initial findings are expected to be released in summer 2021.
The clinical trial follows a government decision, backed by the Joint Committee on Vaccination and Immunisation, to delay the second doses of both the Pizer/BioNTech and University of Oxford/AstraZeneca vaccines from 21 days to 12 weeks after the first dose.
However, the Department of Health and Social Care said that, if the clinical trial showed promising results, the government may consider reviewing the vaccine regimen approach if needed, “but only if proven to be safe and recommended by the Joint Committee on Vaccination and Immunisation”.
“This is a hugely important clinical trial that will provide us with more vital evidence on the safety of these vaccines when used in different ways,” said Nadhim Zahawi, the minister for COVID-19 vaccine deployment.
“Nothing will be approved for use more widely than the study, or as part of our vaccine deployment programme, until researchers and the regulator are absolutely confident the approach is safe and effective.
“This is another great step forward for British science, expertise and innovation, backed by government funding — and I look forward to seeing what it produces.”
Gino Martini, chief scientist at the Royal Pharmaceutical Society, said: “I welcome this very important government-backed study, which will help healthcare practitioners make informed decisions about the potential for alternating different vaccines — again the UK is leading the way on clinical science and innovation.”
This study is separate to the COVID-19 national immunisation programme and vaccines are not being mixed as part of rollout of the national COVID-19 immunisation programme.
Patients will be recruited over the course of February via the NHS COVID-19 Vaccine Research Registry, with vaccinations expected to start towards the middle of the month.
The eight arms of the clinical trial include:
- Two doses of the Oxford/AstraZeneca vaccine at 28 days apart;
- Two doses of the Oxford/AstraZeneca vaccine at 12 weeks apart — as a control group;
- Two doses of the Pfizer/BioNTech vaccine at 28 days apart;
- Two doses of the Pfizer/BioNTech vaccine at 12 weeks apart — as a control group;
- The Oxford/AstraZeneca vaccine for the first dose, followed by the Pfizer/BioNTech vaccine for the second, at 28 days apart;
- The Oxford/AstraZeneca vaccine for the first dose, followed by the Pfizer/BioNTech vaccine for the second, at 12 weeks apart;
- The Pfizer/BioNTech vaccine for the first dose, followed by the Oxford/AstraZeneca vaccine for the second, at 28 days apart; and
- The Pfizer/BioNTech vaccine for the first dose, followed by the Oxford/AstraZeneca vaccine for the second, at 12 weeks apart.