Shutterstock.com
Fewer than 20,000 people in England have been prescribed anticholesterol drug inclisiran (Leqvio; Novartis) in primary care since 2021, despite expectations set by the NHS that 300,000 people would be taking the drug by 2024.
A freedom of information request sent by The Pharmaceutical Journal to the NHS Business Services Authority (NHS BSA), revealed that 19,416 people were prescribed inclisiran in primary care in England in the three years between October 2021 and October 2024.
This prescribing rate is far below expectations set by NHS England in September 2021, when it announced a “world first” deal with Novartis to make inclisiran available to patients from GP surgeries, estimating that around 300,000 people would receive the drug in its first three years.
The low prescribing levels follow concerns raised by the Royal College of General Practitioners (RCGP) and the British Medical Association (BMA) after the drug was approved by the National Institute of Health and Care Excellence (NICE).
At the time, the RCGP and BMA urged caution about prescribing inclisiran because they said long-term data on cardiovascular outcomes and safety were not available.
When asked to comment on the latest prescribing data, both organisations told The Pharmaceutical Journal that their positions on inclisiran remain the same as in 2021, pending long-term trial data that are expected in 2026.
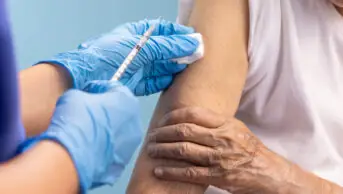
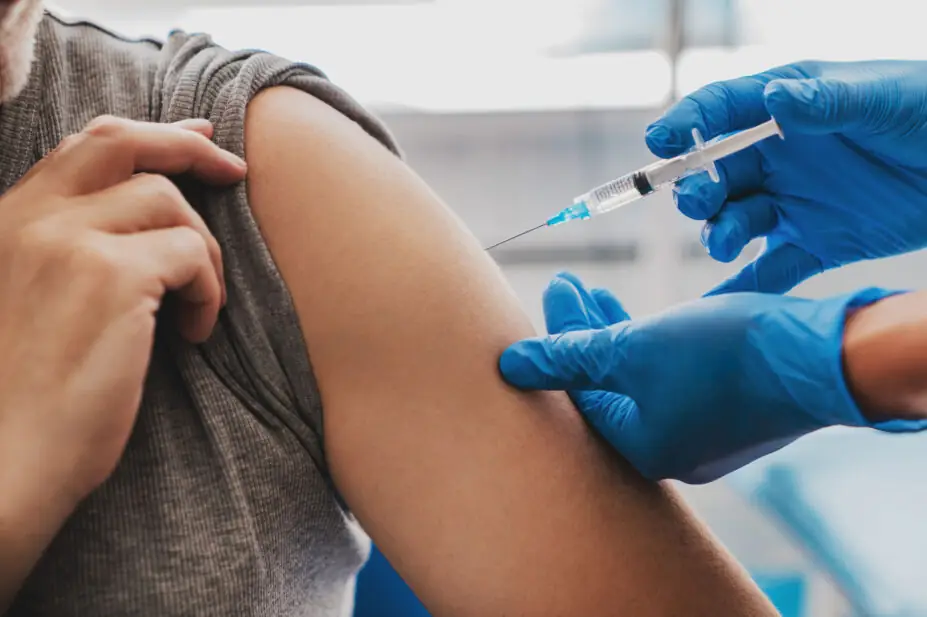
Inclisiran is a type of cholesterol-lowering treatment that uses RNA interference to boost the liver’s ability to remove harmful cholesterol from the blood.
It can be given in primary care as a twice-yearly injection and is approved by NICE for secondary prevention of cardiovascular disease (CVD) for those with persistently high low-density lipoprotein cholesterol, despite taking statins with or without other lipid-lowering medicines. Inclisiran can also be offered when people cannot take statins.
Helen Kilminster, pharmacist advanced clinical practitioner and deputy primary care network clinical director at Citrus Health Primary Care Network (PCN), said: “Hesitation in adoption by primary care is inevitable as administration of inclisiran is not a national core contracted service offered by GPs and collective action by GPs is currently in force.
“As a PCN, we have been cautious with delivering this service to balance sustainable resource and capacity within our team.
“Fundamentally, our PCN cares for a patient population within a high social-economic deprivation area and identified high prevalence of CVD. Inclisiran allows us to offer a better service for lipid management to ensure better CVD outcomes,” she added.
While inclisiran prescribing has not yet met NHS England’s estimations, the data from NHS BSA showed that the number has been rising month on month, with 3,231 patients prescribed inclisiran in October 2024.
Danny Bartlett, clinical lead at Kent, Surrey and Sussex Primary Care School, told The Pharmaceutical Journal that he is encouraged by the steady progress on inclisiran prescribing, adding that pharmacy teams play a vital role in identifying and treating these patients to target.
“I think more needs to be done to proactively look for patients with raised cholesterol, rather than opportunistically coming across the results in routine blood tests,” he said.
Commenting on the NHS data, a spokesperson for Novartis said: “Our records indicate over 35,000 patients have received inclisiran since it was approved by NICE in October 2021, and we continue to work closely with NHS England to support its clinically-led roll out to eligible CVD patients.”
Novartis clarified that the 35,000 figure includes patients who have been prescribed inclisiran in secondary care.
A spokesperson for NHS England said: “Inclisiran is an effective, NICE-recommended drug that provides an additional option for NHS clinicians to prescribe alongside other treatments, such as blood thinners, that reduce the risk of CVD.”
An investigation by The Pharmaceutical Journal into NICE’s approval of inclisiran previously revealed an “extremely unusual” level of political influence on the independent body to give the green light to the drug quickly.