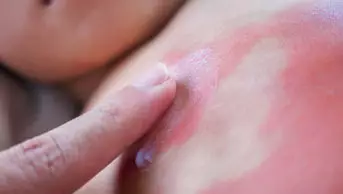
Patients taking expectorants containing ambroxol or bromhexine have a small increased risk of severe skin reactions such as Stevens-Johnson syndrome or erythema multiforme, says the European Medicine Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) following a safety review.
The expectorants, available over the counter and widely used outside the UK, are traditionally given to thin the mucus of patients with lung disease.
The PRAC has recommended that a new side effect warning is added to product information.
The review was prompted by original concerns raised by Belgium’s medicines safety regulator following reports of allergic reactions and severe skin reactions with ambroxol. The review was extended to include bromhexine-containing products as bromhexine can be converted into ambroxol in the body.
The revised product information, which includes a general warning about the small possibility of a severe allergic reaction, will also apply to lozenges containing ambroxol for treating sore throats.
The new warnings will also appear in information for ambroxol formulations offered via injection to prevent and treat lung complications post-surgery, as well as to treat respiratory distress syndrome in premature or newborn babies or to increase lung development after birth.