Shutterstock.com
After reading this CPD article you should be able to:
- Identify the risk factors for adverse drug reactions (ADRs) in children and young people;
- Understand the factors that impact ADR-associated pharmacokinetics in children and young people;
- Identify ADRs associated with the treatment of various childhood conditions, including asthma, infections and allergies;
- Appropriately assess and manage paediatric ADRs.
Adverse drug reactions (ADRs) are defined by the World Health Organization (WHO) as “a response to a drug which is noxious, and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease, or for the modification of physiological function”[1]. Children comprise around 20% of the UK population but use only 5% of the prescribed medications[2,3]. However, a Canadian study demonstrated that the proportion of children receiving prescribed medicines is not uniform, with 25% of children accounting for more than 70% of prescriptions[4].
This article aims to review ADRs in children and young people, highlighting the main differences from the adult population, focusing on important disease groups — such as asthma and infection — and steps that can be taken to appropriately manage ADRs.
ADR risk in children
The British National Formulary for Children (BNFc) provides medicines guidance for children up to the day before their 18th birthday[5]. In UK paediatric practice, most centres will aim to transition a young person to adult services in line with this, although some of the most complex patients, for example those with complex neurodisabilities requiring multidisciplinary care, may remain in paediatric services for longer[6,7].
Historically, children were believed to be at a lower risk of ADRs than adults[8–10]. However, studies now show that children are at an equal, if not greater, risk of ADRs compared with non-elderly adults[8,11]. A systematic review of paediatric ADRs identified 30 studies, detailing incidence rates of ADRs causing hospital admission; the rates ranged from 0.4–10.3%[12]. Prospective data from a secondary/tertiary centre in the UK determined that 2.9% of all acute paediatric admissions (n=8,345) were directly related to an ADR[13]. Internationally, studies have shown that 0.6–16.8% of paediatric inpatients experience an ADR, while prospective data for the UK shows that 17.7% experience at least one ADR[12,14]. In outpatient and community settings, a similar proportion of ADRs have been reported, ranging from 0.6–11.0%[12]. More recent studies have demonstrated no significant change in the rates of ADRs in children over the past decade[15–17].
The known risk factors for ADRs in children are:
- Polypharmacy[14,17,18];
- Previous adverse reaction to another drug[8,17,18];
- Female sex[8,13,18];
- Impaired liver or renal function[8,18];
- General anaesthetic use[14,17,18];
- Off-label and unlicensed drug use[18,19];
- Genetic polymorphisms[8,18].
Pharmacists, as medicines experts, should counsel patients on potential ADRs, and also have a role in the identification, management and reporting of ADRs. Opportunities to perform these activities exist in both the inpatient setting (e.g. during ward rounds), and the outpatient setting (e.g. at the point of drug dispensing).
Identification of ADRs in children and young people can be particularly challenging. This, in part, relates to the population, as babies and young children have limited communication. This is reflected in the low rates of reporting of suspected ADRs in UK neonatal units, where despite more than 100,000 babies being cared for annually, less than 10 Yellow Card reports are received by the Medicines and Healthcare products Regulatory Agency (MHRA) per year[20,21]. However, even in neonatal units, where active surveillance is undertaken, it is possible to increase the reporting rate[22]. The presence of pharmacists — even in highly specialised areas, such as neonatal and paediatric intensive care units — is critical for improved recognition and reporting of ADRs.
Why are ADRs different in children?
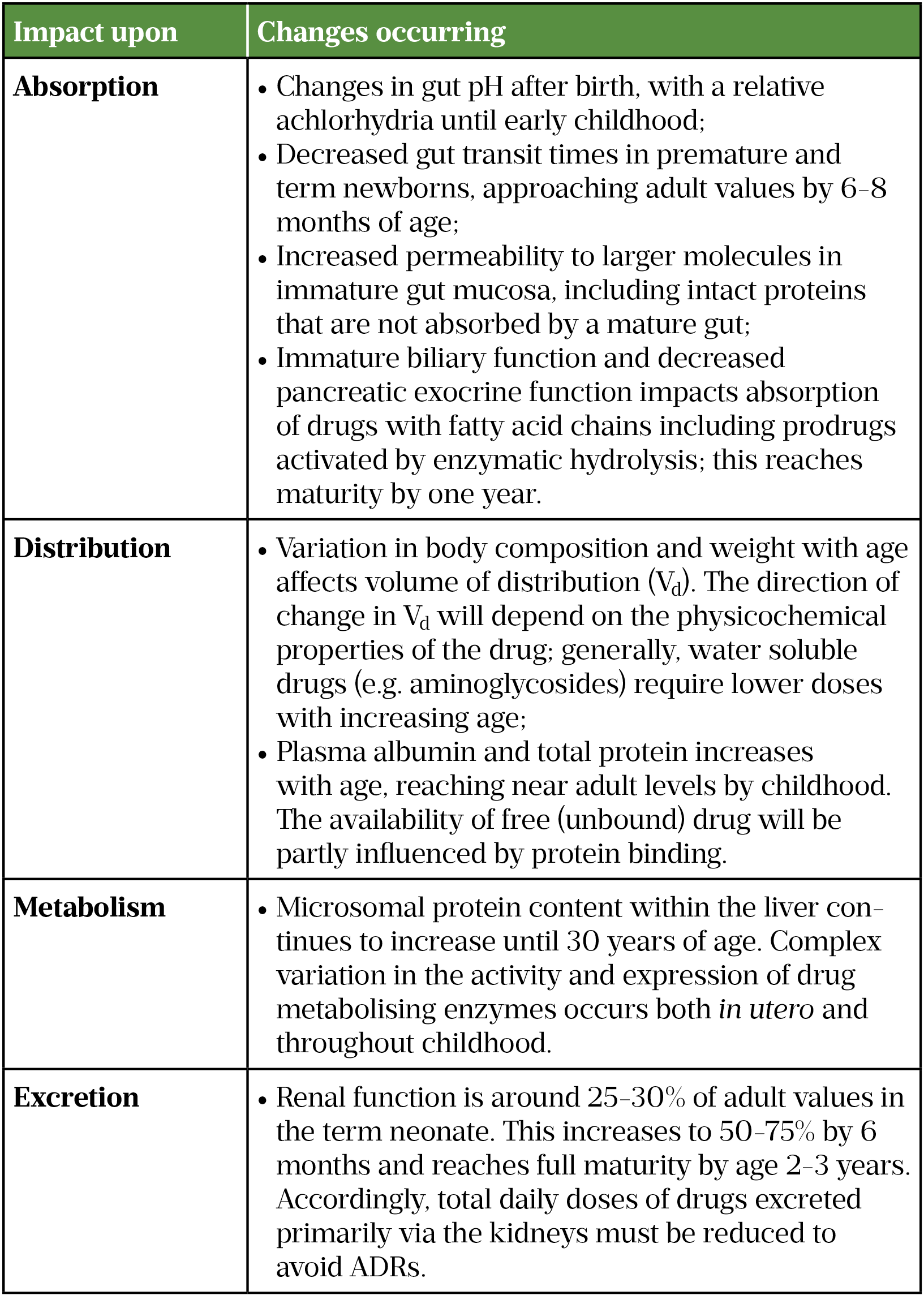
One of the challenges of describing ADRs in children is the rapid development that alters their anatomy and physiology[23]. The diseases often experienced by children differ from adults, and manifestations of diseases that occur in both childhood and adulthood may also be different[23]. The most rapid changes in pharmacokinetics occur in the first year of life[24]. By the age of one year, body weight will have tripled and body surface area doubled[5,23]. The relative proportions of fat, water and protein also change rapidly during infancy. Total body water (TBW) between birth and one year decreases from 80% to 65%. As the proportion of TBW decreases, the percentage of body fat increases, almost tripling by one year of age[25]. In contrast, protein mass does not start to increase significantly until the second year of life, when infants become more ambulatory[23]. These changes, and their potential impact on drug pharmacokinetics, are summarised in Table 1.
Types of ADRs
The Rawlins and Thompson classification of ADRs include two principal types: type A reactions, which are dose-dependent and predictable (e.g. bleeding and anticoagulants); and type B reactions, which are non-dose-dependent and not predictable (e.g. penicillin hypersensitivity)[27]. Over time, this classification system has been extended to include: type C, dose- and time-dependent (chronic) reactions (e.g. corticosteroids and adrenal insufficiency); type D, delayed reactions (e.g. thalidomide and phocomelia); type E, withdrawal reactions (e.g. withdrawal seizures on discontinuation of anticonvulsants) and type F, failure of therapy[28].
Common mechanisms of ADRs include:
- Synergistic effects between drug combinations (e.g. use of analgesic combinations post-operatively, specifically the combination of opioids and other analgesics such as non-steroidal anti-inflammatories [NSAIDs] or paracetamol)[29].
- Antagonistic effects between drug combinations (e.g. concurrent use of antiepileptic drugs lamotrigine and carbamazepine can result in reduced levels of lamotrigine)[5,30].
- Abnormal pharmacokinetics from genetic polymorphisms in drug-metabolizing enzymes (e.g. phase I enzyme cytochrome P450 2D6 [CYP2D6] has 141 different alleles described to date, representing one of the most well understood examples of how pharmacogenetic variation can influence drug metabolism)[31,32].
Recognising ADRs in children
In the paediatric population, availability of high-quality data about the harms of medicines used can be an issue. For more established medicines, there may be limited clinical trial data supporting their use in children and young people, making recognition of potential ADRs more difficult. The lack of paediatric-specific drugs also means that off-label and unlicensed use of medicines is common in paediatrics; this type of medication use is also associated with increased ADR risk[19,33]. Following the introduction of the Paediatric Regulation in the EU in 2007, the situation has improved, with 238 new medicines for use in children authorised between 2007 and 2015[34].
Unrecognised ADRs that appear in the post-marketing surveillance stage of drug development may compound the issue of limited information on paediatric drugs. Across a 25-year period examining 548 drugs newly approved for use in adults and children by the United States Food and Drug Administration, 45 (8.2%) acquired new black box warnings and 16 (2.9%) were withdrawn from the market[35]. Half of these occurred more than seven years after the drug was introduced to the market[35]. In the UK, this data is captured by spontaneous reporting of suspected ADRs through the MHRA’s Yellow Card scheme[36]. The majority of reports are submitted by healthcare professionals, although the proportion of reports submitted by patients, parents and carers has been steadily increasing as a result of several public health campaigns[37–39]. While spontaneous reporting systems have their advantages, and are one of WHO’s five minimum requirements for a functional national pharmacovigilance system, they have their drawbacks, the principal of which is enormous under-reporting, up to 98%, of ADRs[20,40–42]. Reasons given for under-reporting are numerous and include indifference, fear of litigation and ignorance on how to report ADRs[43–45].
Initial observation of ADRs
In the UK, the routine immunisation schedule begins from the age of eight weeks[46]. Therefore, vaccinations represent one of the most commonly used medicinal products in those aged under one year[20,47]. As such, it is also this age group that generates large numbers of reports for suspected ADRs for vaccinations, dwarfing the number reported for medicines[20,47,48].
The most common ADRs reported for vaccinations include pyrexia, injection site reactions and headache[48]. As the vast majority of children and young people receiving a vaccination are healthy, any symptom exhibited may be considered a suspected ADR. The vaccines with the most reported ADRs in children and young people, submitted via the UK Yellow Card scheme, include the human papilloma virus (HPV) vaccines (Cervarix [GlaxoSmithKline UK], Gardasil and Gardasil 9 [Merck Sharp & Dohme Limited]), the measles, mumps and rubella (MMR) vaccine and the meningococcal B vaccine (Bexsero [GlaxoSmithKline UK]), with estimated reporting rates of 48.2, 36.6 and 19.3 per 100,000 doses respectively in 2017/2018[48].
In the UK, the categorisation and management of suspected ADRs related to vaccines is detailed in ‘Immunisation against infectious disease’, popularly known as The Green Book[49]. For common adverse events following immunisations (AEFI), such as fever and local site reactions, simple self-care advice is recommended[49–51]. Rare AEFIs are typically neurologically or immune-mediated and include seizures, idiopathic thrombocytopaenic purpura and anaphylaxis; incidence rates are typically less than 1 per 100,000 doses[52–54]. In these cases, the appropriate emergency guidelines should be followed and referral to an allergist is usually indicated before deciding whether additional doses of the same vaccine or vaccines containing the same components should be used (for more on management of anaphylaxis, see here)[49,55].
Common childhood conditions and associated ADRs
Asthma
Asthma is the most common, non-infective, chronic disease of childhood, affecting one in 11 children and young people in the UK[56]. Mainly managed in primary care, the management of asthma is guided by national and international evidence-based guidelines[57–59]. Commonly used medications include beta-agonists, inhaled corticosteroids (ICS), leukotriene receptor antagonists, xanthine derivatives and, more recently, monoclonal antibodies[5]. Each group of medications has its own adverse effect profile (see Table 2).
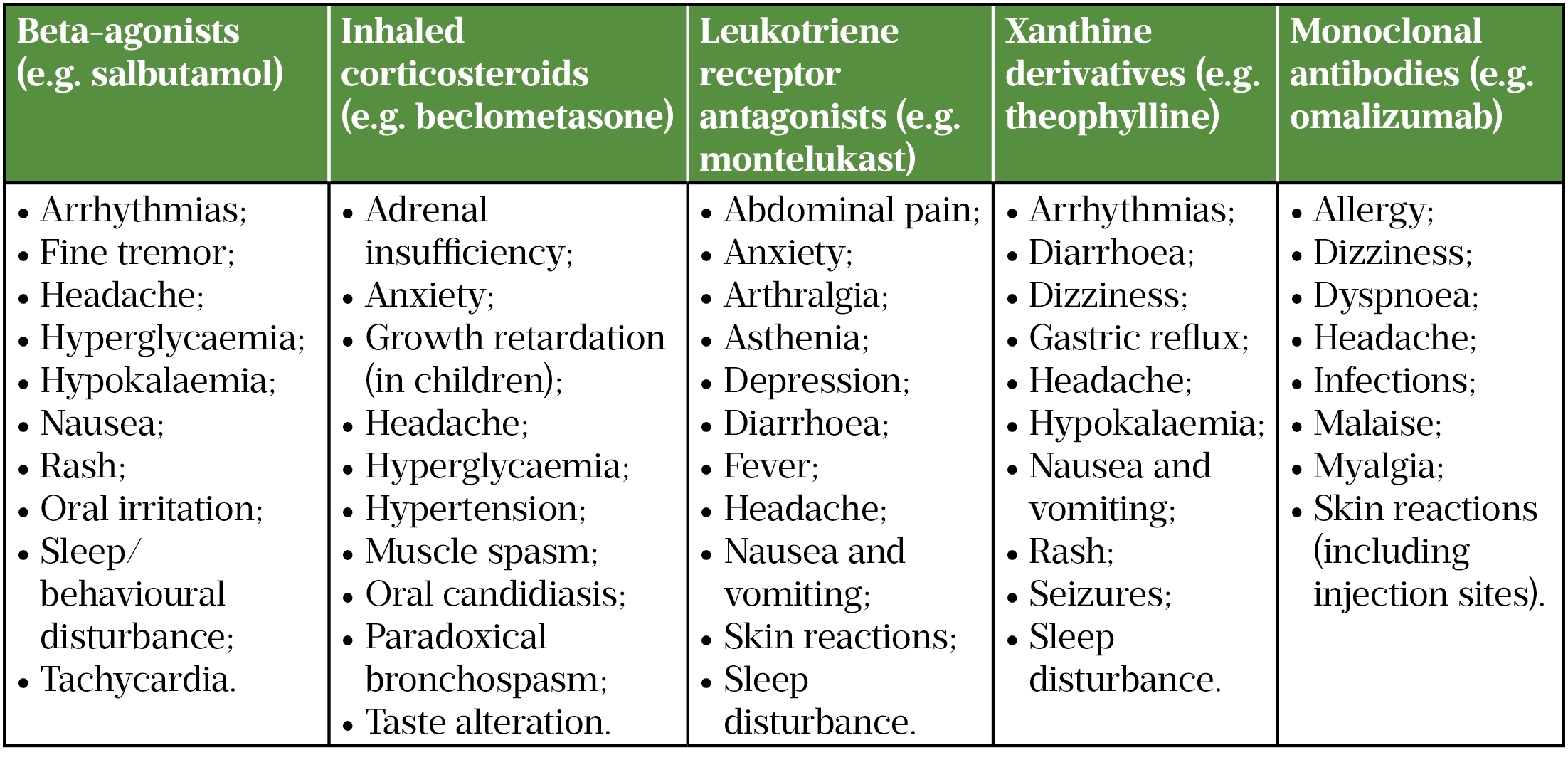
A systematic review of ADRs associated with asthma medications commonly used in children found that, of the ADRs reviewed, 43% (n=174) were associated with ICS, with serious ADRs ranging from 1–7% per drug across all medication classes[62]. Use of corticosteroids is common in asthma treatment[58,63]. Children and young people typically receive lower doses of ICS, meaning that some systemic ADRs to corticosteroids, such as striae and fat redistribution, are rarely seen in children and young people with asthma[64]. However, there are still concerns about ADRs caused by ICS in children and young people, particularly adrenal suppression[65–67]. Adrenal suppression can be difficult to identify, as the presentation can be asymptomatic; subtle (e.g. loss of growth velocity, increased lethargy, weight loss); or florid (e.g. symptomatic adrenal crisis)[66]. Owing to the insidious nature of adrenal suppression, a high level of vigilance is required, to ensure that it is considered in children and young people using corticosteroids (inhaled and oral), and that asthma medications are actively managed so children and young people are on the lowest dose that allows for symptom control[66,67].
Infection
After vaccines, anti-infective agents are the most commonly reported group of medicines for ADRs in children and young people (all age groups), with antibacterials accounting for around half of the ADR burden in children[12,68–70]. The most common ADRs reported are nausea, vomiting, diarrhoea and skin rashes[12]. Beta-lactams are reportedly responsible for causing up to 40% of these ADRs[69].
Up to 6% of children are now labelled as allergic to beta-lactam antibiotics, with three-quarters of these children being labelled before their third birthday[71,72]. If these labels are then carried forward into adulthood, it can perpetuate the use of non-beta-lactam antibacterials, resulting in increased costs, reduced efficacy, increased antibacterial resistance and potentially increased mortality[73–75]. Indeed in a large programme of research on ADRs in children and young people, parental anxiety about the potential future implications of ADRs in their child(ren) was well highlighted[76]. Nevertheless, in studies where children have been tested for drug allergy or undergone drug challenges, more than 90% of children were able to tolerate the antibacterial they had previously been deemed allergic to[71,77,78].
The Royal College of Paediatrics and Child Health has produced a care pathway for drug allergy and describes a set of competencies required to diagnose, treat and optimally manage drug allergy[79]. In the context of suspected drug allergy with only mild reactions, where no additional treatment is required, drug provocation testing is gold standard and has become routine among large centres in the UK and elsewhere[80].
Allergy
Systemic antihistamines are commonly used in children for symptomatic relief in a variety of allergic diseases, including allergic rhinitis, allergic conjunctivitis, food allergy, chronic spontaneous urticaria and atopic eczema[5,81]. Studies have shown that, of the major drug classes, antihistamines are responsible for around 1–3% of reported ADRs[82–84].
Historically, although highly effective, first-generation antihistamines (e.g. chlorphenamine and promethazine), were associated with a greater incidence of ADRs than second-generation antihistamines, such as loratadine and cetirizine[85]. de Vries and van Hunsel examined reports of ADRs from systemic antihistamines in the Netherlands and found 228 reports over a 13-year period, 5 of which were deemed serious[81]. This included one death, which was attributed to malignant neuroleptic malignancy syndrome after alimemazine was prescribed for sedative purposes. Interestingly, the remaining four serious ADRs were seen in second-generation antihistamines. One report included a boy aged 14 years who had an atrioventricular re-entry tachycardia after co-administration of azithromycin and fexofenadine; and three children who had convulsions after receiving loratadine/desloratadine[81]. A similar report reviewing ADRs from antihistamines used in children and young people found that, of 8,918 reports, 57% involved second-generation antihistamines[86]. Of the serious ADRs, classified as causing death, hospitalisation, life-threatening, permanent disability and birth defects, there was no significant difference between first- and second-generation antihistamines.
Other more commonly reported ADRs attributed to antihistamines include skin reactions, headache, somnolence and aggressive behaviour[61,81,87].
Pain
Pain is often undertreated in children and young people, and its management varies globally[88–90]. Three groups of analgesics are commonly used in children and young people: paracetamol, NSAIDs and opioids. These account for around 10% of ADRs reported in children[12,91]. A systematic review examined ADRs involving these analgesics in children presenting with acute pain[92]. Twenty-three studies involving 2,300 children were included. Opioids were associated with markedly more adverse events than paracetamol or NSAIDs, particularly in the central nervous system (CNS), and dual therapy with a non-opioid/opioid combination conferred a protective effect for ADRs compared with opioids alone[92]. Codeine raised particular concerns about harms in children after the deaths/serious harms reported in children given ‘standard’ doses of codeine, whom were later found to be ultra-rapid metabolisers at the CYP2D6 locus[93,94]. Codeine is now contraindicated in children and young people aged under 18 years who undergo procedures for obstructive sleep apnoea, and is not routinely prescribed in children[5,95]. With the known implications of CYP2D6 polymorphisms on codeine use — in conjunction with the improved access to and reduced costs of genotyping — it is possible that the evolving field of pharmacogenomics will allow safer use of codeine and other opioids in perioperative pain control in children and young people[96].
Epilepsy
Epilepsy is a common neurological condition affecting both adults and children. The majority of patients will depend on antiepileptic drugs (AEDs) to achieve seizure control[97–99]. AEDs are the second most frequently reported therapeutic class associated with ADRs in children and young people and up to 25% of AED treatment failure has been attributed to ADRs[12,97,100]. The risk of ADRs from AEDs is almost tripled in patients prescribed more than one drug[101]. Sodium valproate and carbamazepine account for the majority of AED ADRs; however, this is likely to be because of their widespread use among children and young people with epilepsy[101–103]. The most common ADRs reported involve the CNS and include behavioural disturbance, somnolence/fatigue and increased seizures[12,97,101–103]. While it is recognised that most AEDs increase the risk of harm to the foetus, valproate is of particular concern, with up to 40% of children exposed affected by neurodevelopmental disorders in later childhood[104,105]. As such, valproate is now only prescribed to female patients post puberty with a pregnancy prevention programme in place[106]. Pharmacists have an essential role in the identification of women and girls of childbearing potential and provision of information via this scheme in the UK (see here for more information)[106].
Diabetes
Insulin is the primary agent used to treat diabetes mellitus in children[5]. Various insulin products are available that vary in their time-action profiles: short-acting (including soluble insulin and rapid-acting insulin), intermediate-acting insulins and long-acting insulins[5].
Hypoglycaemia is the most serious and potentially life threatening adverse effect of insulin and a major barrier to achieving adequate glycaemic control[107]. Risk factors for hypoglycaemia include longer duration of disease, renal impairment and impaired hypoglycaemia awareness[108–110]. For children and young people, older age is associated with a lower risk of severe hypoglycaemia[111]. Educational awareness should be improved in children and young people who have a higher risk of hypoglycaemic consequences. Additionally, a higher, more relaxed glycated haemoglobin (HbA1c) target can be recommended[112–115]. When comparing the different insulin preparations, rapid-acting insulin analogues and insulin glargine result in fewer hypoglycaemic events when compared with regular and neutral protamine Hagedorn insulins respectively[107,116,117].
Insulin is an anabolic hormone involved in promoting the uptake of fatty acid into adipose tissue; unsurprisingly, with the exception of insulin detemir, weight gain is another commonly reported adverse effect of insulin[61,118,119]. Although the mechanisms are not entirely understood, detemir is associated with reduced food intake compared with other insulin preparations[120,121]. Weight gain can be a significant barrier to adherence to insulin, especially in a teenage population[122,123].
Several types of local injection site lesions from subcutaneous insulin have been reported. True hypersensitivity reactions are uncommon[107]. Other lesions include lipoatrophy, lipohypertrophy and cutaneous amyloidosis[124]. The latter two conditions can both appear as painless, slow-growing subcutaneous nodules at insulin injection sites[124]. Patients are advised to rotate their injection sites within the same body area to reduce the risk of nodule formation and reduced insulin absorption[5].
Attention deficient hyperactive disorder
Attention deficit hyperactivity disorder (ADHD) is now the most common neurodevelopmental disorder, with a global prevalence of 5%[125]. In the UK, methylphenidate and lisdexamfetamine are recommended in children aged over five years where ADHD symptoms persist despite environmental modifications[126]. Both agents can only be initiated by an appropriate specialist trained in the diagnosis, management and monitoring of ADHD[5,126]. Second-line medications include atomoxetine and guanfacine[126].
A review of 43 studies involving 7,244 children treated with amphetamine (n=1,076), methylphenidate (n=2,092), atomoxetine (n=3127) and modafinil (n=949) revealed ADRs as one of the most common reasons for non-completion of treatment in the trial setting[127]. For all four drugs, anorexia, gastrointestinal pain and headache were the most commonly reported ADRs[127].
Reasons for discontinuation of ADHD medications have been reviewed among various age groups, identifying female sex and the presence of co-morbid psychiatric conditions as risk factors[128].
According to UK Yellow Card scheme reports for methylphenidate and lisdexamfetamine, the age group with the largest number of reported ADRs was 10–19 years, with a drop in the number of submitted reports in each subsequent age group[61]. It is not known if this reflects the peak years where medication is used for this condition, or increased susceptibility to ADRs in the teenage years.
Corticosteroids
ICS have been discussed previously; however, systemic corticosteroids (e.g. prednisolone, methylprednisolone and dexamethasone) are also widely used in paediatrics for their anti‐inflammatory, immunomodulatory and immunosuppressive characteristics across a range of diseases[129]. Equally wide ranging are the potential adverse effects of corticosteroids on almost all organ systems[130].
Retrospective reviews have demonstrated that both dose and duration of therapy are independent predictors of ADRs secondary to use of corticosteroids[131,132]. Increasingly, genetic polymorphisms, such as the single nucleotide polymorphism rs591118 in the platelet-derived growth factor gene (PDGFD), are being implicated in the type and severity of ADRs experienced by patients requiring corticosteroids[60,133].
Prevention and monitoring are essential to reduce the incidence of serious ADRs in patients requiring corticosteroids. It is recommended that patients are routinely asked about ADRs related to corticosteroids throughout therapy and individual risk factors should also be taken into consideration. Particular focus should be given to avoid[134]:
- Opportunistic infection;
- Loss of bone mineral density and osteoporosis;
- Hyperglycaemia and steroid-induced diabetes mellitus;
- Adrenal insufficiency;
- Growth suppression.
National and international agreement on how best to monitor and manage patients requiring long-term systemic corticosteroids is limited[135]. Nevertheless, evidence-based, practical recommendations on how best to manage the complications of systemic corticosteroid therapy exist for both adults and children[136].
Other specialist areas where ADRs are common
Biologic agents are increasingly used across most paediatric subspecialties[137,138]. While a detailed review of biologics lies outside the scope of this article, previous work has highlighted that despite being used routinely in paediatric practice for more than two decades, only 60% of biologics have a licensed paediatric indication[139]. This again highlights the difficulties in obtaining information about potential harms in children and young people.
Childhood cancers are rare, with one in 500 children developing cancer by the age of 14 years in the UK[140]. The majority of treatment regimens involve use of multiple drugs in varying combinations at high doses with broad toxicity profiles, resulting in a wide variety of complications[141]. As such, ADRs seen in paediatric cancers are beyond the scope of this article and have been previously dealt with in previous reviews, most recently by Conyers et al.[141].
Actions pharmacists can take to manage ADRs
When managing ADRs in primary and secondary care, the WHO recommends three steps for evaluation[142]:
- Assess the nature and severity of the reaction;
- Establish the cause;
- Take corrective and follow-up action.
The first step in managing an ADR, even before assessing its nature and severity, is to suspect that one exists in the first place. This can be difficult in a population where communication skills may be limited by age or by conditions such as neurodevelopmental delay. The task of identification therefore often falls on the parents/guardians. However, this identification relies on the parent/guardian and/or child knowing what signs and symptoms to look for. Unfortunately, parents/guardians often feel that clinicians do not provide them with enough advice regarding ADRs[76]. The patient information leaflet (PIL) for Calpol (Johnson and Johnson) has more than 1,900 words and a Flesch Reading Ease of 58.2, making it “fairly difficult to read”[143,144]. Although certainly not suitable for children, some parents/guardians will also have difficulty reading PILs, which makes communication of potential ADRs to both the parents/guardians and the child vital. The Medicines for Children website can be a useful resource for parents/guardians wanting more accessible medicines information[145]. Other additional resources that can be helpful in finding information about known ADRs can be found in the Box.
Box: Additional resources for information about known adverse drug reactions
- British National Formulary for Children[5]
- MHRA Yellow Card scheme Interactive Drug Analysis Profiles[61]
- Electronic medicines compendium[146]
- The International Encylopedia of Adverse Drug Reactions and Interactions[147]
Medicines use reviews (MURs) are less frequently performed in children, with one study showing that 81% of pharmacists had not performed a MUR in patients aged under 18 years[148]. Given that the purpose of these reviews is to improve patients’ understanding of their medicines; highlight problematic side effects and propose solutions, where appropriate; improve adherence; and reduce medicines wastage, MURs would help identify and educate children taking multiple medicines on ADRs[149].
Assessing the nature and severity of the reaction and establishing the cause
Historically, none of the causality tools for assessing ADRs — such as the Naranjo probability scale — were designed for use in paediatrics[150–152]. The Adverse Drug Reactions in Children (ADRIC) programme highlighted the difficulties in applying these existing tools within paediatric populations and the need to develop paediatric-specific methodologies[153]. The ADRIC tools developed include the Liverpool Causality Assessment Tool (LCAT) and the Liverpool Avoidability Assessment Tool (LAAT), both based on existing tools but with more user-friendly flow diagrams[153]. The LCAT classifies ADRs as “unlikely”, “possible”, “probable” or “definite” and LAAT classifies them as “unassessable”, “not avoidable”, “possibly avoidable” and “definitely avoidable”[152,154]. Assessment of ADRs by healthcare professionals is often informal, which can lead to inconsistencies in practice. The LCAT and LAAT offer a systematic approach to assessment.
When assessing causality, healthcare professionals should be mindful that the ADR may be secondary to the excipient and not necessarily the drug. Excipient toxicity is well recognised in the neonate[155]. Arthur and Burgess illustrate several examples of ‘problem’ excipients[156].
Severity assessments describe the clinical impact of ADRs. The Hartwig scale is commonly used; severity levels range from Level 1 (the ADR required no change in treatment with the suspected drug) to Level 7 (the ADR was fatal)[157].
Taking corrective and follow-up action
Once an ADR is suspected, management will depend on the severity of the ADR and how important that medicine is to the child’s treatment. A patient prescribed systemic anti-cancer therapy to treat a life-limiting condition will be prepared for more severe ADRs, and on balance of risk/benefit will likely proceed with treatment. This would not be the same for a patient prescribed a medicine for sleep, for example. Similarly, a pharmacist may not be confident in advising that a patient’s systemic anti-cancer medicine is paused, unless the ADR was an immediate threat to life. Whether a drug should be discontinued depends on several factors: is it dose-related; the severity of reaction; the consequences of stopping the medicine; satisfactory disease control with the offending drug; and the ability to treat the ADR[158].
If the ADR is dose-related, the drug may be stopped or paused to allow concentrations to decrease before commencing a more appropriate dose or immediately switched to the most appropriate dose. If the ADR is not felt to be dose-related, then there are three options in paediatrics: to stop the drug; continue with the drug and add in a second medicine to manage the effects of the ADR; or switch the drug to another in class. The second option, although possibly the easiest, will increase polypharmacy burden and risk additional ADRs[159]. Regardless of the corrective action chosen, communication between multi-disciplinary team members, including the patient or parents/guardians themselves, is vital.
For hospital-based pharmacists, the management of ADRs is often easier, as they have direct access to the prescriber. This is especially true in paediatrics, where most long-term medicines in children are initiated by specialist paediatricians. For community pharmacists, the management of ADRs can be more difficult and the need to contact the GP or paediatrician will have to be decided on a case-by-case basis. Development of links to hospital pharmacists, prescribers and medication safety officers can help facilitate communication about potential ADRs[153].
Consideration should always be given to reporting the ADR to the MHRA via the Yellow Card scheme. Parents involved in the ADRIC study described confusion and uncertainty about the roles and responsibilities for recording and reporting of suspected ADRs[76]. Most parents and children were unaware that such a system existed. Those that did report an ADR via the Yellow Card Scheme did so because they wanted to help prevent other children experiencing the same ADRs and realised that their report would not directly help their own child[76].
The role of children themselves should not be forgotten. They may want to report the ADR given that they are the ones affected, and children as young as 10 years have been known to submit their own Yellow Card[86]. Barriers to ADR reporting by children and young people are not well understood and represent an area of unmet need which must be addressed before development of new strategies aimed at improving reporting by children and young people[86].
Conclusion
ADRs are common occurrences in everyday clinical practice and should be at the forefront of the minds of pharmacists working with children and young people.
Actionable points that pharmacists can take into their everyday clinical practice include:
- Suspect ADRs — ask your patients/parents/guardians about both positive and negative responses to medication. Consider whether requests for over-the-counter medicines could be owing to ADRs to their current treatment;
- Assess whether an ADR is likely to have occurred (use LCAT or LAAT);
- Take corrective action;
- Report the ADR(s) — use the Yellow Card scheme;
- Educate your patients about possible ADRs. Use the medicines for children PILs to identify ADRs that are specifically relevant to children.
Financial and conflicts of interest disclosure
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was used in the production of this manuscript.
References
- 1Safety of medicines: a guide to detecting and reporting adverse drug reactions: why health professionals need to take action. World Health Organization. Quality Assurance and Safety of Medicines Team 2002.
- 2Overview of the UK population: August 2019. Office for National Statistics. 2019.https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/august2019 (accessed Apr 2021).
- 3Prescriptions dispensed in the community statistics for 1997 to 2007: England. NHS Digital. 2008.https://digital.nhs.uk/data-and-information/publications/statistical/prescriptions-dispensed-in-the-community/prescriptions-dispensed-in-the-community-statistics-for-england-1997-2007 (accessed Apr 2021).
- 4Khaled LA, Ahmad F, Brogan T, et al. Prescription medicine use by one million Canadian children. Paediatrics & Child Health 2001;8:6A-56A. doi:10.1093/pch/8.suppl_a.6a
- 5BNF for Children. London: : BMJ Group, Pharmaceutical Press, and RCPCH Publications 2020. http://www.medicinescomplete.com (accessed Apr 2021).
- 6Best practice examples of health transition. Royal College of Paediatrics and Child Health. https://www.rcpch.ac.uk/resources/best-practice-examples-health-transition (accessed Apr 2021).
- 7From the pond into the sea. Children’s transition to adult health services. Care Quality Commission. 2014.https://www.cqc.org.uk/sites/default/files/CQC_Transition%20Report_Summary_lores.pdf (accessed Apr 2021).
- 8Rieder M. New Ways to Detect Adverse Drug Reactions in Pediatrics. Pediatric Clinics of North America 2012;59:1071–92. doi:10.1016/j.pcl.2012.07.010
- 9Martin RM, Biswas PN, Freemantle SN, et al. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. British Journal of Clinical Pharmacology 1998;46:505–11. doi:10.1046/j.1365-2125.1998.00817.x
- 10Pouyanne P. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. BMJ 2000;320:1036–1036. doi:10.1136/bmj.320.7241.1036
- 11Cammack B, Oschman A, Lewis T. Improving Recognition and Reporting of Adverse Drug Reactions in the NICU: A Quality Improvement Project. Pediatric Quality & Safety 2019;4:e203. doi:10.1097/pq9.0000000000000203
- 12Smyth RMD, Gargon E, Kirkham J, et al. Adverse Drug Reactions in Children—A Systematic Review. PLoS ONE 2012;7:e24061. doi:10.1371/journal.pone.0024061
- 13Gallagher RM, Mason JR, Bird KA, et al. Adverse Drug Reactions Causing Admission to a Paediatric Hospital. PLoS ONE 2012;7:e50127. doi:10.1371/journal.pone.0050127
- 14Thiesen S, Conroy EJ, Bellis JR, et al. Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children – a prospective observational cohort study of 6,601 admissions. BMC Med 2013;11. doi:10.1186/1741-7015-11-237
- 15Lombardi N, Crescioli G, Bettiol A, et al. Characterization of serious adverse drug reactions as cause of emergency department visit in children: a 5-years active pharmacovigilance study. BMC Pharmacol Toxicol 2018;19. doi:10.1186/s40360-018-0207-4
- 16Nasso C, Mecchio A, Rottura M, et al. A 7-Years Active Pharmacovigilance Study of Adverse Drug Reactions Causing Children Admission to a Pediatric Emergency Department in Sicily. Front Pharmacol 2020;11. doi:10.3389/fphar.2020.01090
- 17Andrade PHS, Santos A da S, Souza CAS, et al. Risk factors for adverse drug reactions in pediatric inpatients: a systematic review. Therapeutic Advances in Drug Safety 2017;8:199–210. doi:10.1177/2042098617702615
- 18Rieder M. Adverse Drug Reactions in Children: Pediatric Pharmacy and Drug Safety. The Journal of Pediatric Pharmacology and Therapeutics 2019;24:4–9. doi:10.5863/1551-6776-24.1.4
- 19Cuzzolin L, Atzei A, Fanos V. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opinion on Drug Safety 2006;5:703–18. doi:10.1517/14740338.5.5.703
- 20Hawcutt DB, Russell N-J, Maqsood H, et al. Spontaneous adverse drug reaction reports for neonates and infants in the UK 2001-2010: content and utility analysis. Br J Clin Pharmacol 2016;82:1601–12. doi:10.1111/bcp.13067
- 21NDAU 2016 Report. Neonatal Data Analysis Unit (NDAU). 2017.https://www.imperial.ac.uk/media/imperial-college/medicine/dept-medicine/infectious-diseases/neonatology/NDAU-2016-Report-v1.1-(002).pdf (accessed Apr 2021).
- 22Roberts EK, Hawcutt DB, Turner MA. Prospective identification and causality evaluation of suspected adverse drug reactions in neonates. Br J Clin Pharmacol 2020;87:1541–6. doi:10.1111/bcp.14485
- 23Rational Therapeutics for Infants and Children. National Academies Press 2000. doi:10.17226/9816
- 24Kearns GL. Impact of developmental pharmacology on pediatric study design: Overcoming the challenges. Journal of Allergy and Clinical Immunology 2000;106:S128–38. doi:10.1067/mai.2000.109419
- 25Toro-Ramos T, Paley C, Pi-Sunyer FX, et al. Body composition during fetal development and infancy through the age of 5 years. Eur J Clin Nutr 2015;69:1279–89. doi:10.1038/ejcn.2015.117
- 26Anderson GD. Children Versus Adults: Pharmacokinetic and Adverse-Effect Differences. Epilepsia 2002;43:53–9. doi:10.1046/j.1528-1157.43.s.3.5.x
- 27Rawlins M, Thompson J. Mechanisms of adverse drug reactions. In: Textbook of Adverse Drug Reactions. Oxford: : Oxford University Press 1991. N/A.
- 28Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. The Lancet 2000;356:1255–9. doi:10.1016/s0140-6736(00)02799-9
- 29Pasternak GW. Preclinical Pharmacology and Opioid Combinations. Pain Med 2012;13:S4–11. doi:10.1111/j.1526-4637.2012.01335.x
- 30Czuczwar SJ, Kaplanski J, Swiderska-Dziewit G, et al. Pharmacodynamic interactions between antiepileptic drugs: preclinical data based on isobolography. Expert Opinion on Drug Metabolism & Toxicology 2009;5:131–6. doi:10.1517/17425250802677826
- 31CYP2D6. Pharmacogene Variation Consortium. 2020.https://www.pharmvar.org/gene/CYP2D6 (accessed Apr 2021).
- 32Weinshilboum R. Inheritance and Drug Response. N Engl J Med 2003;348:529–37. doi:10.1056/nejmra020021
- 33Conroy S. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ 2000;320:79–82. doi:10.1136/bmj.320.7227.79
- 3410-year Report to the European Commission. General report on the experience acquired as a result of the application of the Paediatric Regulation. European Medicines Agency. 2016.https://www.ema.europa.eu/en/human-regulatory/overview/paediatric-medicines/paediatric-regulation#ten-year-report-section (accessed Apr 2021).
- 35Lasser KE. Timing of New Black Box Warnings and Withdrawals for Prescription Medications. JAMA 2002;287:2215. doi:10.1001/jama.287.17.2215
- 36About Yellow Card. Medicines & Healthcare products Regulatory Agency. 2021.https://yellowcard.mhra.gov.uk/the-yellow-card-scheme/ (accessed Apr 2021).
- 37Yellow Card Scheme. Medicines & Healthcare products Regulatory Agency. 2020.https://yellowcard.mhra.gov.uk/ (accessed Apr 2021).
- 38Foy M. Patient ADR Reporting – UK View. Medicines & Healthcare products Regulatory Agency. 2013.https://www.ema.europa.eu/en/documents/presentation/presentation-adverse-drug-reaction-reporting-united-kingdom-view-mick-foy_en.pdf (accessed Apr 2021).
- 39Harrison K, Goldsmith C. Board Meeting. Adverse Drug Reaction (ADR) reporting by patient. Medicines & Healthcare products Regulatory Agency. 2019.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/852275/Item_08__ADR_reporting_by_patients.pdf (accessed Apr 2021).
- 40WHO pharmacovigilance indicators: a practical manual for the assessment of pharmacovigilance systems. World Health Organization. 2015.https://www.who.int/medicines/areas/quality_safety/safety_efficacy/EMP_PV_Indicators_web_ready_v2.pdf?ua (accessed Apr 2021).
- 41Fletcher A. Spontaneous adverse drug reaction reporting vs event monitoring: a comparison. J R Soc Med 1991;84:341–4.https://www.ncbi.nlm.nih.gov/pubmed/2061900
- 42van der Heijden PGM, van Puijenbroek EP, van Buuren S, et al. On the assessment of adverse drug reactions from spontaneous reporting systems: the influence of under-reporting on odds ratios. Statist Med 2002;21:2027–44. doi:10.1002/sim.1157
- 43Varallo FR, Guimarães S de OP, Abjaude SAR, et al. Causes for the underreporting of adverse drug events by health professionals: a systematic review. Rev esc enferm USP 2014;48:739–47. doi:10.1590/s0080-623420140000400023
- 44Hazell L, Shakir SAW. Under-Reporting of Adverse Drug Reactions. Drug Safety 2006;29:385–96. doi:10.2165/00002018-200629050-00003
- 45Gahr M, Eller J, Connemann BJ, et al. Underreporting of adverse drug reactions: Results from a survey among physicians. Eur psychiatr 2017;41:S369–S369. doi:10.1016/j.eurpsy.2017.02.377
- 46Complete routine immunisation schedule. Public Health England. 2020.https://www.gov.uk/government/publications/the-complete-routine-immunisation-schedule (accessed Apr 2021).
- 47Hawcutt DB, Mainie P, Riordan A, et al. Reported paediatric adverse drug reactions in the UK 2000-2009. British Journal of Clinical Pharmacology 2012;73:437–46. doi:10.1111/j.1365-2125.2011.04113.x
- 48Vaccine-associated suspected adverse reactions reported via the Yellow Card Scheme during 2018. Medicines and Healthcare products Regulatory Agency. 2019.https://www.whatdotheyknow.com/request/690857/response/1653809/attach/5/JCVI%202019.pdf?cookie_passthrough=1 (accessed Apr 2021).
- 49Vaccine safety and adverse events following immunisation: the green book. In: Immunisation Against Infectious Disease. London, UK: : Public Health England 2013. N/A.
- 50Fever in under 5s: assessment and initial management. NICE Clinical Guidance [CG143]. National Institute for Health and Care Excellence. 2019.http://www.nice.org.uk/guidance/cg143 (accessed Apr 2021).
- 51Ishaque SM, Khosruzzaman SM, Ahmed DS, et al. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol 2018;18. doi:10.1186/s12876-018-0788-9
- 52McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. Journal of Allergy and Clinical Immunology 2016;137:868–78. doi:10.1016/j.jaci.2015.07.048
- 53Lafaurie M, Baricault B, Lapeyre-Mestre M, et al. Risk of Vaccine-Induced Immune Thrombocytopenia in Children. Nationwide Case Cross-over and Self-Controlled Case Series Studies in France. Blood 2018;132:738–738. doi:10.1182/blood-2018-99-110522
- 54Febrile seizures and childhood vaccines. Centers for Disease Control and Prevention. 2020.https://www.cdc.gov/vaccinesafety/concerns/febrile-seizures.html (accessed Apr 2021).
- 55Allergy care pathway for anaphylaxis. Royal College of Paediatrics and Child Health. 2011.https://www.rcpch.ac.uk/resources/allergy-care-pathway-anaphylaxis (accessed Apr 2021).
- 56Time to take action on asthma. Asthma UK. 2014.https://www.asthma.org.uk/globalassets/campaigns/compare-your-care-2014.pdf (accessed Apr 2021).
- 57Bloom CI, Nissen F, Douglas IJ, et al. Exacerbation risk and characterisation of the UK’s asthma population from infants to old age. Thorax 2017;73:313–20. doi:10.1136/thoraxjnl-2017-210650
- 58British guideline on the management of asthma (SIGN 158). SIGN. 2019.https://www.sign.ac.uk/media/1773/sign158-updated.pdf (accessed Apr 2021).
- 59Pocket guide for asthma management and prevention (for adults and children older than 5 years). Global Initiative for Asthma. 2020.https://ginasthma.org/wp-content/uploads/2020/04/Main-pocket-guide_2020_04_03-final-wms.pdf (accessed Apr 2021).
- 60King C, McKenna A, Farzan N, et al. Pharmacogenomic associations of adverse drug reactions in asthma: systematic review and research prioritisation. Pharmacogenomics J 2020;20:621–8. doi:10.1038/s41397-019-0140-y
- 61Interactive Drug Analysis Profiles. Yellow Card. 2020.https://yellowcard.mhra.gov.uk/iDAP/ (accessed Apr 2021).
- 62Leung JS, Johnson DW, Sperou AJ, et al. A systematic review of adverse drug events associated with administration of common asthma medications in children. PLoS ONE 2017;12:e0182738. doi:10.1371/journal.pone.0182738
- 63Reddel HK, FitzGerald JM, Bateman ED, et al. GINA 2019: a fundamental change in asthma management. Eur Respir J 2019;53:1901046. doi:10.1183/13993003.01046-2019
- 64Lapi F, Kezouh A, Suissa S, et al. The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur Respir J 2012;42:79–86. doi:10.1183/09031936.00080912
- 65Patel L. Symptomatic adrenal insufficiency during inhaled corticosteroid treatment. Archives of Disease in Childhood 2001;85:330–4. doi:10.1136/adc.85.4.330
- 66Paton J. Adrenal responses to low dose synthetic ACTH (Synacthen) in children receiving high dose inhaled fluticasone. Archives of Disease in Childhood 2006;91:808–13. doi:10.1136/adc.2005.087247
- 67Hawcutt DB, Jorgensen AL, Wallin N, et al. Adrenal responses to a low-dose short synacthen test in children with asthma. Clin Endocrinol 2014;82:648–56. doi:10.1111/cen.12655
- 68Lovegrove MC, Geller AI, Fleming-Dutra KE, et al. US Emergency Department Visits for Adverse Drug Events From Antibiotics in Children, 2011–2015. Journal of the Pediatric Infectious Diseases Society 2018;8:384–91. doi:10.1093/jpids/piy066
- 69Rosli R, Dali AF, Aziz NAbd, et al. Reported Adverse Drug Reactions in Infants: A Nationwide Analysis in Malaysia. Front Pharmacol 2017;8. doi:10.3389/fphar.2017.00030
- 70Iftikhar S, Sarwar MR, Saqib A, et al. Causality and preventability assessment of adverse drug reactions and adverse drug events of antibiotics among hospitalized patients: A multicenter, cross-sectional study in Lahore, Pakistan. PLoS ONE 2018;13:e0199456. doi:10.1371/journal.pone.0199456
- 71Rebelo Gomes E, Fonseca J, Araujo L, et al. Drug allergy claims in children: from self-reporting to confirmed diagnosis. Clin Exp Allergy 2007;0:071119182754009-??? doi:10.1111/j.1365-2222.2007.02870.x
- 72Vyles D, Chiu A, Simpson P, et al. Parent-Reported Penicillin Allergy Symptoms in the Pediatric Emergency Department. Academic Pediatrics 2017;17:251–5. doi:10.1016/j.acap.2016.11.004
- 73Norton AE, Konvinse K, Phillips EJ, et al. Antibiotic Allergy in Pediatrics. Pediatrics 2018;141:e20172497. doi:10.1542/peds.2017-2497
- 74Charneski L, Deshpande G, Smith SW. Impact of an Antimicrobial Allergy Label in the Medical Record on Clinical Outcomes in Hospitalized Patients. Pharmacotherapy 2011;31:742–7. doi:10.1592/phco.31.8.742
- 75Blumenthal KG, Lu N, Zhang Y, et al. Recorded Penicillin Allergy and Risk of Mortality: a Population-Based Matched Cohort Study. J GEN INTERN MED 2019;34:1685–7. doi:10.1007/s11606-019-04991-y
- 76Smyth R, Peak M, Turner M. Families’ experiences of suspected adverse drug reactions: implications for communication and pharmacovigilance. In: ADRIC: Adverse Drug Reactions In Children – a programme of research using mixed methods. Southampton, UK: : NIHR Journals Library 2014. N/A.
- 77Vyles D, Adams J, Chiu A, et al. Allergy Testing in Children With Low-Risk Penicillin Allergy Symptoms. Pediatrics 2017;140:e20170471. doi:10.1542/peds.2017-0471
- 78Vezir E, Erkocoglu M, Civelek E, et al. The evaluation of drug provocation tests in pediatric allergy clinic: A single center experience. allergy asthma proc 2014;35:156–62. doi:10.2500/aap.2014.35.3744
- 79Allergy care pathway for drug allergy. Royal College of Paediatrics and Child Health. 2011.https://www.rcpch.ac.uk/resources/allergy-care-pathway-drug-allergy (accessed Apr 2021).
- 80Marrs T, Fox AT, Lack G, et al. The diagnosis and management of antibiotic allergy in children: Systematic review to inform a contemporary approach. Arch Dis Child 2014;100:583–8. doi:10.1136/archdischild-2014-306280
- 81de Vries TW, van Hunsel F. Adverse drug reactions of systemic antihistamines in children in the Netherlands. Arch Dis Child 2016;101:968–70. doi:10.1136/archdischild-2015-310315
- 82Čirko-Begović A, Vrhovac B, Bakran I. Intensive monitoring of adverse drug reactions in infants and preschool children. Eur J Clin Pharmacol 1989;36:63–5. doi:10.1007/bf00561025
- 83Kaushal R, Goldmann DA, Keohane CA, et al. Adverse Drug Events in Pediatric Outpatients. Ambulatory Pediatrics 2007;7:383–9. doi:10.1016/j.ambp.2007.05.005
- 84Menniti-lppolito F, Raschetti R, Da Cas R, et al. Active monitoring of adverse drug reactions in children. The Lancet 2000;355:1613–4. doi:10.1016/s0140-6736(00)02219-4
- 85Ten Eick AP, Blumer JL, Reed MD. Safety of Antihistamines in Children. Drug Safety 2001;24:119–47. doi:10.2165/00002018-200124020-00003
- 86Bhoombla N, Preston J, Ainsworth J, et al. Pharmacovigilance Reports Received from Children and Young People, and Development of Information to Aid Future Reporting from this Age Group. Pediatr Drugs 2020;22:335–41. doi:10.1007/s40272-020-00391-6
- 87Woods CG, Rylance ME, Cullen RE, et al. Adverse reactions to drugs in children. BMJ 1987;294:869–70. doi:10.1136/bmj.294.6576.869-a
- 88Habich M, Wilson D, Thielk D, et al. Evaluating the Effectiveness of Pediatric Pain Management Guidelines. Journal of Pediatric Nursing 2012;27:336–45. doi:10.1016/j.pedn.2011.06.002
- 89Stevens BJ, Yamada J, Estabrooks CA, et al. Pain in hospitalized children: Effect of a multidimensional knowledge translation strategy on pain process and clinical outcomes. Pain 2014;155:60–8. doi:10.1016/j.pain.2013.09.007
- 90Petrack EM, Christopher NC, Kriwinsky J. Pain Management in the Emergency Department: Patterns of Analgesic Utilization. PEDIATRICS 1997;99:711–4. doi:10.1542/peds.99.5.711
- 91Silva DCB, Araujo OR, Arduini RG, et al. Adverse drug events in a paediatric intensive care unit: a prospective cohort. BMJ Open 2013;3:e001868. doi:10.1136/bmjopen-2012-001868
- 92Hartling L, Ali S, Dryden DM, et al. How Safe Are Common Analgesics for the Treatment of Acute Pain for Children? A Systematic Review. Pain Research and Management 2016;2016:1–15. doi:10.1155/2016/5346819
- 93Ciszkowski C, Madadi P, Phillips MS, et al. Codeine, Ultrarapid-Metabolism Genotype, and Postoperative Death. N Engl J Med 2009;361:827–8. doi:10.1056/nejmc0904266
- 94Gasche Y, Daali Y, Fathi M, et al. Codeine Intoxication Associated with Ultrarapid CYP2D6 Metabolism. N Engl J Med 2004;351:2827–31. doi:10.1056/nejmoa041888
- 95Codeine for analgesia: restricted use in children because of reports of morphine toxicity. Medicines and Healthcare products Regulatory Agency. 2014.https://www.gov.uk/drug-safety-update/codeine-for-analgesia-restricted-use-in-children-because-of-reports-of-morphine-toxicity (accessed Apr 2021).
- 96Chidambaran V, Sadhasivam S, Mahmoud M. Codeine and opioid metabolism. Current Opinion in Anaesthesiology 2017;30:349–56. doi:10.1097/aco.0000000000000455
- 97Lee J. Antiepileptic Drugs in Children : Current Concept. J Korean Neurosurg Soc 2019;62:296–301. doi:10.3340/jkns.2019.0099
- 98Epilepsy in childhood. Epilepsy Society. 2018.https://www.epilepsysociety.org.uk/epilepsy-childhood (accessed Apr 2021).
- 99Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disorders 2015;17:117–23. doi:10.1684/epd.2015.0736
- 100Kwan P, Brodie MJ. Early Identification of Refractory Epilepsy. N Engl J Med 2000;342:314–9. doi:10.1056/nejm200002033420503
- 101Anderson M, Egunsola O, Cherrill J, et al. A prospective study of adverse drug reactions to antiepileptic drugs in children. BMJ Open 2015;5:e008298–e008298. doi:10.1136/bmjopen-2015-008298
- 102Egunsola O, Choonara I, Sammons HM, et al. Safety of antiepileptic drugs in children and young people: A prospective cohort study. Seizure 2018;56:20–5. doi:10.1016/j.seizure.2018.01.018
- 103Suman A, Gosavi DeveshD. Study of adverse drug effects of antiepileptic drugs used in pediatric patients in a tertiary care rural hospital – a Pharmacovigilance study. JYP 2017;9:60–4. doi:10.5530/jyp.2017.9.12
- 104Antiepileptic drugs: review of safety of use during pregnancy. Medicines and Healthcare products Regulatory Agency. 2021.https://www.gov.uk/government/publications/public-assesment-report-of-antiepileptic-drugs-review-of-safety-of-use-during-pregnancy/antiepileptic-drugs-review-of-safety-of-use-during-pregnancy#:~:text=The%20overarching%20findings%20of%20the,compared%20with%20the%20general%20population (accessed Apr 2021).
- 105Coste J, Blotiere P-O, Miranda S, et al. Risk of early neurodevelopmental disorders associated with in utero exposure to valproate and other antiepileptic drugs: a nationwide cohort study in France. Sci Rep 2020;10. doi:10.1038/s41598-020-74409-x
- 106Valproate Pregnancy Prevention Programme: actions required now from GPs, specialists, and dispensers. Medicines and Healthcare products Regulatory Agency. 2018.https://www.gov.uk/drug-safety-update/valproate-pregnancy-prevention-programme-actions-required-now-from-gps-specialists-and-dispensers (accessed Apr 2021).
- 107Donner T, Sarkar S. Insulin – pharmacology, therapeutic regimens, and principles of intensive insulin therapy. In: Endotext. MA, USA: : MDText.com, Inc 2000. N/A.
- 108Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444–b5444. doi:10.1136/bmj.b5444
- 109Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140–7. doi:10.1007/s00125-007-0599-y
- 110Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev 2004;20:479–86. doi:10.1002/dmrr.482
- 111Karges B, Rosenbauer J, Kapellen T, et al. Hemoglobin A1c Levels and Risk of Severe Hypoglycemia in Children and Young Adults with Type 1 Diabetes from Germany and Austria: A Trend Analysis in a Cohort of 37,539 Patients between 1995 and 2012. PLoS Med 2014;11:e1001742. doi:10.1371/journal.pmed.1001742
- 1126. Glycemic Targets:Standards of Medical Care in Diabetes—2018. Dia Care 2017;41:S55–64. doi:10.2337/dc18-s006
- 113Type 2 diabetes in adults: management. NICE guidance [NG28]. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ng28/chapter/1-recommendations#blood-glucose-management-2 (accessed Apr 2021).
- 114Type 1 diabetes in adults: diagnosis and management. NICE guidance [NG17]. National Institute for Health and Care Excellence. 2020. https://www.nice.org.uk/guidance/ng17/chapter/1-Recommendations#blood-glucose-management-2 (accessed Apr 2021).
- 115Abraham MB, Jones TW, Naranjo D, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes 2018;19:178–92. doi:10.1111/pedi.12698
- 116Brunelle RL, Llewelyn J, Anderson JH, et al. Meta-Analysis of the Effect of Insulin Lispro on Severe Hypoglycemia in Patients With Type 1 Diabetes. Diabetes Care 1998;21:1726–31. doi:10.2337/diacare.21.10.1726
- 117Holcombe JH, Zalani S, Arora VK, et al. Comparison of insulin lispro with regular human insulin for the treatment of type 1 diabetes in adolescents. Clinical Therapeutics 2002;24:629–38. doi:10.1016/s0149-2918(02)85138-4
- 118Meneghini LF, Rosenberg KH, Koenen C, et al. Insulin detemir improves glycaemic control with less hypoglycaemia and no weight gain in patients with type 2 diabetes who were insulin naive or treated with NPH or insulin glargine: clinical practice experience from a German subgroup of the PREDICTIVE study. Diabetes Obes Metab 2007;9:418–27. doi:10.1111/j.1463-1326.2006.00674.x
- 119Rosenstock J, Davies M, Home PD, et al. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408–16. doi:10.1007/s00125-007-0911-x
- 120Zachariah S, Sheldon B, Shojaee-Moradie F, et al. Insulin Detemir Reduces Weight Gain as a Result of Reduced Food Intake in Patients With Type 1 Diabetes. Diabetes Care 2011;34:1487–91. doi:10.2337/dc11-0098
- 121Zafar MI, Hu C, Liu D, et al. Insulin Detemir Causes Lesser Weight Gain in Comparison to Insulin Glargine: Role on Hypothalamic NPY and Galanin. Journal of Diabetes Research 2014;2014:1–7. doi:10.1155/2014/458104
- 122Wisting L, Froisland DH, Skrivarhaug T, et al. Disturbed Eating Behavior and Omission of Insulin in Adolescents Receiving Intensified Insulin Treatment: A nationwide population-based study. Diabetes Care 2013;36:3382–7. doi:10.2337/dc13-0431
- 123Wisting L, Reas DL, Bang L, et al. Eating patterns in adolescents with type 1 diabetes: Associations with metabolic control, insulin omission, and eating disorder pathology. Appetite 2017;114:226–31. doi:10.1016/j.appet.2017.03.035
- 124Ansari AM, Osmani L, Matsangos AE, et al. Current insight in the localized insulin-derived amyloidosis (LIDA): clinico-pathological characteristics and differential diagnosis. Pathology – Research and Practice 2017;213:1237–41. doi:10.1016/j.prp.2017.08.013
- 125Young S, Hollingdale J, Absoud M, et al. Guidance for identification and treatment of individuals with attention deficit/hyperactivity disorder and autism spectrum disorder based upon expert consensus. BMC Med 2020;18. doi:10.1186/s12916-020-01585-y
- 126Attention deficit hyperactivity disorder: diagnosis and management. NICE guidance [NG87]. National Institute for Health and Care Excellence. 2018.https://www.nice.org.uk/guidance/ng87/resources/attention-deficit-hyperactivity-disorder-diagnosis-and-management-pdf-1837699732933 (accessed Apr 2021).
- 127Aagaard L, Hansen EH. The occurrence of adverse drug reactions reported for attention deficit hyperactivity disorder (ADHD) medications in the pediatric population: a qualitative review of empirical studies. NDT 2011;:729. doi:10.2147/ndt.s26403
- 128Khan MU, Aslani P. A Review of Factors Influencing the Three Phases of Medication Adherence in People with Attention-Deficit/Hyperactivity Disorder. Journal of Child and Adolescent Psychopharmacology 2019;29:398–418. doi:10.1089/cap.2018.0153
- 129Ferrara G, Petrillo M, Giani T, et al. Clinical Use and Molecular Action of Corticosteroids in the Pediatric Age. IJMS 2019;20:444. doi:10.3390/ijms20020444
- 130Saag K, Furst D. Major side effects of systemic glucocorticoids. UpToDate. 2020.https://www.uptodate.com/contents/major-side-effects-of-systemic-glucocorticoids#H5 (accessed Apr 2021).
- 131Huscher D, Thiele K, Gromnica-Ihle E, et al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis 2009;68:1119–24. doi:10.1136/ard.2008.092163
- 132Curtis JR, Westfall AO, Allison J, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 2006;55:420–6. doi:10.1002/art.21984
- 133Hawcutt DB, Francis B, Carr DF, et al. Susceptibility to corticosteroid-induced adrenal suppression: a genome-wide association study. The Lancet Respiratory Medicine 2018;6:442–50. doi:10.1016/s2213-2600(18)30058-4
- 134Hoes JN, Jacobs JWG, Boers M, et al. EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases. Annals of the Rheumatic Diseases 2007;66:1560–7. doi:10.1136/ard.2007.072157
- 135Wong J, Shaikh M, Mason A, et al. Adrenal suppression secondary to exogenous glucocorticoid – guidance for children on long term steroid therapy. Greater Glasgow & Clyde Paediatric Guidelines. 2017.https://www.clinicalguidelines.scot.nhs.uk/nhsggc-paediatric-clinical-guidelines/nhsggc-guidelines/endocrinology/adrenal-suppression-secondary-to-exogenous-glucocorticoid-guidance-for-children-on-long-term-steroid-therapy/ (accessed Apr 2021).
- 136Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy, Asthma & Clinical Immunology 2013;9:30. doi:10.1186/1710-1492-9-30
- 137Growth of biologics and biosimilars will drive above-average demand for a range of ingredients through 2023, Forecasts Kline. Biosimilar Development. 2019.https://www.biosimilardevelopment.com/doc/growth-of-biologics-and-biosimilars-will-drive-above-average-demand-0001 (accessed Apr 2021).
- 138Niehues T, Özgür TT. The Efficacy and Evidence-Based Use of Biologics in Children and Adolescents. Deutsches Aerzteblatt Online Published Online First: 18 October 2019. doi:10.3238/arztebl.2019.0703
- 139Field M, Boat T. Pediatric Studies of Biologics. In: Safe and effective medicines for children: pediatric studies conducted under the best pharmaceuticals for Children Act and the Pediatric Research Equity Act. DC, USA: : National Academies Press (US) 2012. N/A.
- 140Childhood Cancer Facts & Figures. Children with Cancer UK. 2020.https://www.childrenwithcancer.org.uk/childhood-cancer-info/childhood-cancer-facts-figures/ (accessed Apr 2021).
- 141Conyers R, Devaraja S, Elliott D. Systematic review of pharmacogenomics and adverse drug reactions in paediatric oncology patients. Pediatr Blood Cancer 2017;65:e26937. doi:10.1002/pbc.26937
- 142Drug and Therapeutics Committee Training Course. Management Sciences for Health and World Health Organization. 2007.https://www.who.int/medicines/technical_briefing/tbs/04-PG_Dug-Safety_final-08.pdf?ua=1 (accessed Apr 2021).
- 143Calpol Infant Suspension (GSL). Electronic Medicines Compendium. 2020.https://www.medicines.org.uk/emc/product/260/smpc (accessed Apr 2021).
- 144Spadaro D, Robinson L, Smith L. Assessing readability of patient information materials. Am J Hosp Pharm 1980;37:215–21.https://www.ncbi.nlm.nih.gov/pubmed/7361793
- 145Practical and reliable advice about giving medicine to your child. Medicines for children. 2021.https://www.medicinesforchildren.org.uk/ (accessed Apr 2021).
- 146About emc. Electronic Medicines Compendium. 2010.https://www.medicines.org.uk/emc/about-the-emc (accessed Apr 2021).
- 147Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions. 16th ed. Elsevier Science 2016.
- 148Venables R, Iliopoulou E, Cork T. SP1 Evaluating medicines use reviews (MURs) in children. Arch Dis Child 2020;105:e1.1-e1. doi:10.1136/archdischild-2020-nppg.1
- 149MURs: the basics. What is the Medicines Use Review & Prescription Intervention Service? Pharmaceutical Services Negotiating Committee. 2020.https://psnc.org.uk/services-commissioning/advanced-services/murs/murs-the-basics/ (accessed Apr 2021).
- 150Busto U, Naranjo C, Sellers E. Comparison of two recently published algorithms for assessing the probability of adverse drug reactions. British Journal of Clinical Pharmacology 1982;13:223–7. doi:10.1111/j.1365-2125.1982.tb01361.x
- 151Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. doi:10.1038/clpt.1981.154
- 152Bracken LE, Nunn AJ, Kirkham JJ, et al. Development of the Liverpool Adverse Drug Reaction Avoidability Assessment Tool. PLoS ONE 2017;12:e0169393. doi:10.1371/journal.pone.0169393
- 153Smyth R, Peak M, Turner M, et al. ADRIC: Adverse Drug Reactions In Children – a programme of research using mixed methods. Published Online First: 1 June 2014. doi:10.3310/pgfar02030
- 154Gallagher RM, Kirkham JJ, Mason JR, et al. Development and Inter-Rater Reliability of the Liverpool Adverse Drug Reaction Causality Assessment Tool. PLoS ONE 2011;6:e28096. doi:10.1371/journal.pone.0028096
- 155Valeur KS, Holst H, Allegaert K. Excipients in Neonatal Medicinal Products: Never Prescribed, Commonly Administered. Pharm Med 2018;32:251–8. doi:10.1007/s40290-018-0243-9
- 156How to identify and manage ‘problem’ excipients in medicines for children. The Pharmaceutical Journal Published Online First: 2017. doi:10.1211/pj.2017.20203121
- 157Hartwig S, Siegel J, Schneider P. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229–32.https://www.ncbi.nlm.nih.gov/pubmed/1524068
- 158Scenario: Adverse drug reactions. Clinical Knowledge Summaries. National Institute for Health and Care Excellence. 2017.https://cks.nice.org.uk/topics/adverse-drug-reactions/management/adverse-drug-reactions/ (accessed Apr 2021).
- 159Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ 1997;315:1096–9. doi:10.1136/bmj.315.7115.1096


