Cultura RM / Alamy
Stem cell treatment after high-dose immunosuppressive therapy could be an effective way to manage relapsing-remitting multiple sclerosis, according to interim results from a five-year study[1]
published in JAMA Neurology on 29 December 2014.
Researchers are following the progress of 24 patients with relapsing-remitting multiple sclerosis (RRMS) who received transplants of their own haematopoietic stem cells after receiving high-dose immunosuppressive therapy (HDIT). The phase II study, known as the HALT-MS trial, is testing whether control of inflammation earlier in RRMS may lead to prolonged remission and potentially reverse neurologic dysfunction. After three years without maintenance therapy, most of the patients remained in remission of active disease and had improvements in neurological function.
“Most patients with RRMS who receive approved disease-modifying therapies experience breakthrough disease and accumulate neurologic disability,” say the researchers, led by Richard Nash, of the Colorado Blood Cancer Institute, Denver. “High-dose immunosuppressive therapy with autologous haematopoietic cell transplant may, in contrast, induce sustained remissions in early MS.”
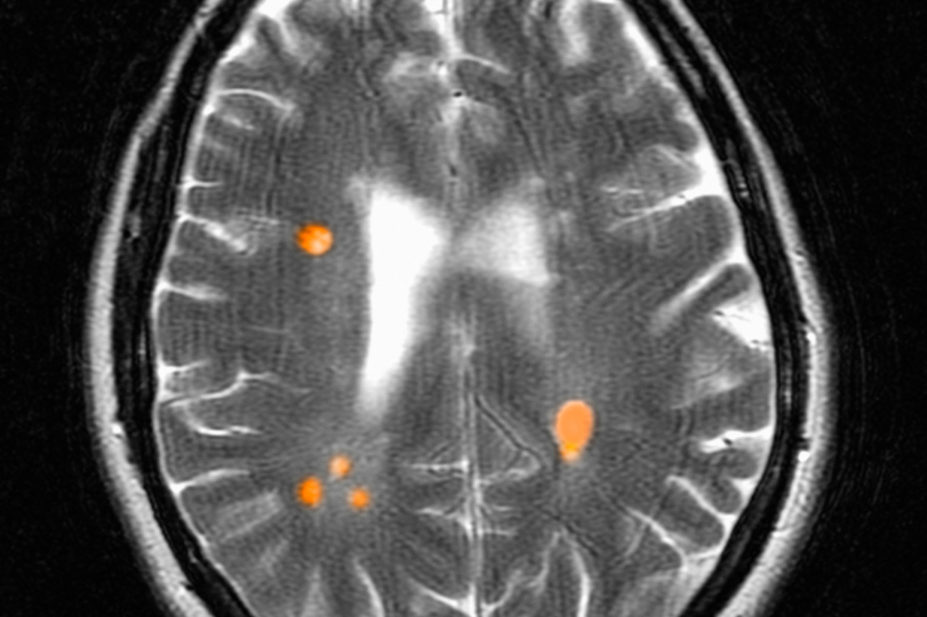
For the 24 patients who received HDIT with autologous haematopoietic cell transplant, the overall rate of event-free survival was 78.4% at three years (90% confidence interval 60.1%-89.0%) — this was defined as survival without loss of neurologic function, clinical relapse or new lesions observed on imaging. Progression-free survival and clinical relapse-free survival were 90.9% (90% CI 73.7%-97.1%) and 86.3% (90% CI 68.1%-94.5%), respectively. Improvements in neurologic disability, quality-of-life and functional scores were also noted.
The researchers report no unexpected side effects. “Most early toxic effects were hematologic and gastrointestinal and were expected and reversible,” they say, but warn that longer follow-up is needed to determine whether the response endures.
The findings are described as promising by the Multiple Sclerosis Trust, a UK charity. “Stem cell therapy for MS holds significant potential but remains at the frontier of clinical research,” says Amy Bowen, the charity’s director of service development.
However, the authors of an editorial[2]
accompanying the JAMA Neurology study say “the jury is still out” on the appropriateness of haematopoietic cell transplant for MS.
“Nash et al show evidence of prolonged depletion of memory CD4+ cells, depletion of CD4+-dominant T-cell receptor clones and evidence of ‘immune reset’; however, clinical or radiologic evidence of relapse trumps immunologic evidence of immune reset, and this study raises concern that those end points have not been adequately achieved.”
References
[1] Nash RA, Hutton GJ, Racke MK et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): a three-year interim report. JAMA Neurology. Published online 29 December 2014. doi:10.1001/jamaneurol.2014.3780.
[2] Paz Soldán MM & Weinshenker BG. Moving targets for hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurology. Published online 29 December 2014. doi:10.1001/jamaneurol.2014.3831.