Bobjgalindo / Wikimedia Commons
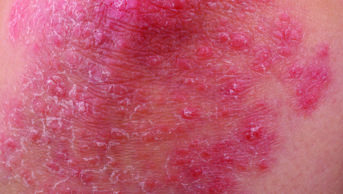
Psoriasis is a chronic inflammatory disease caused by dysregulated immune responses. Recent advances in understanding the underlying cytokine networks has led to attempts to create drugs that can completely clear psoriasis plaques.
In three phase III trials, researchers explored the efficacy of ixekizumab, a monoclonal antibody against interleukin-17a, in a total of 3,866 patients with moderate to severe psoriasis.
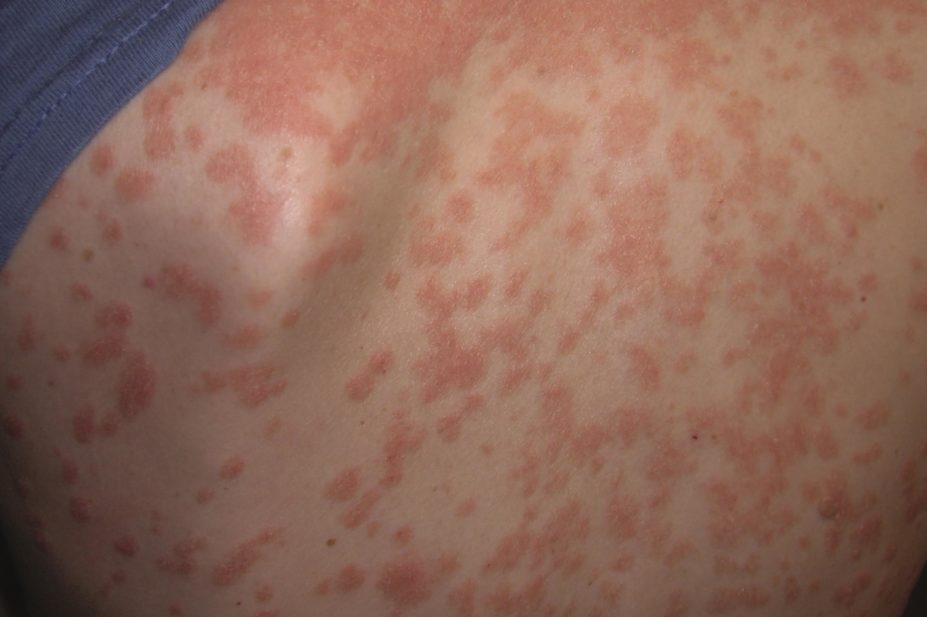
They found that, after 12 weeks, 76.4–81.8% of patients who were randomly assigned to active treatment had minimal or clear psoriasis, compared with 3.2% of patients assigned to placebo. At week 60, 68.7–78.3% of patients who received ixekizumab had maintained their improvement.
In The
New England Journal of Medicine (online, 8 June 2016)[1]
, the researchers say the response rates are some of the highest ever seen from a psoriasis drug, but caution that long-term safety needs assessment.
References
[1] Gordon KB, Blauvelt A, Papp KA et al. Phase III trials of ixekizumab in moderate-to-severe plaque psoriasis. New England Journal of Medicine 2016. doi: 10.1056/NEJMoa1512711