SCIENCE PHOTO LIBRARY
The Medicines Healthcare and Products Regulatory Agency (MHRA) has said it has received 16 reports under its Yellow Card scheme for the use of products that claim to include semaglutide or liraglutide.
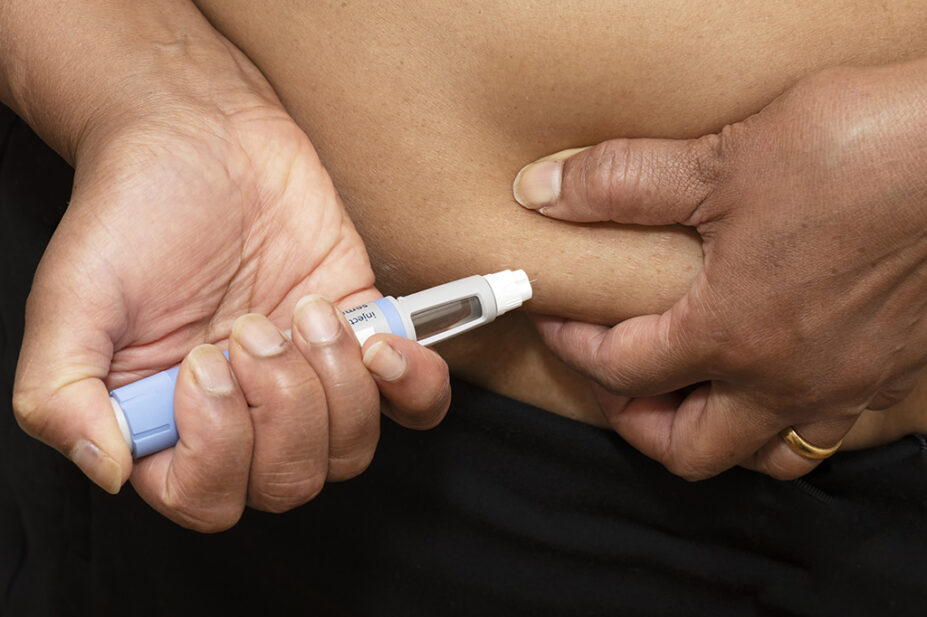
In a drug safety update, published on 23 November 2023, the MHRA said that five of the cases reported that Saxenda (liraglutide; Novo Nordisk) and Ozempic (semaglutide; Novo Nordisk) pens had been confirmed to be falsified with insulin, and some of the recipients “were hospitalised and required urgent care”.
It added that “serious side effects reported in those hospitalised, including hypoglycaemic shock, indicate that the pens may contain insulin rather than semaglutide”.
The safety update said that healthcare professionals should “remain vigilant for symptoms linked to hypoglycaemia in patients who may have obtained a falsified product and provide appropriate treatment for any patient who may have inadvertently administered insulin via these products”.
It also said patients should be urged to check the patient information leaflets for each product to clarify what genuine pens look like.
The MHRA added that Ozempic is a “black triangle medicine”, meaning that, as a new drug, it is more closely monitored and, consequently, all suspected adverse reactions, even those from the legitimate licensed product, should be reported via the Yellow Card scheme.
In October 2023, the MHRA said it had seized 369 potentially fake Ozempic pens between January 2023 and October 2023, and that it had also received reports of fake Saxenda pens in the UK.
In the same month, the MHRA announced that falsified Ozempic pens were identified at two wholesalers in the UK. They highlighted that the falsified pens had German labels and originated from wholesalers in Austria and Germany.
Semaglutide and liraglutide are glucagon-like peptide-1 receptor agonists, which have gained popularity for their use in weight loss.
In March 2023, semaglutide (Wegovy; Novo Nordisk) — which was initially authorised in the UK for use in type 2 diabetes mellitus — was approved as a treatment for weight management by the National Institute for Health and Care Excellence.
You may also be interested in

‘No evidence’ that paracetamol use during pregnancy causes autism, ADHD or intellectual disabilities, meta-analysis finds

Levetiracetam information updated to include studies that suggest no link to autism
