Shutterstock.com
NHS England is to fund a treatment for severe haemophilia that will “dramatically” cut treatment time for thousands of patients.
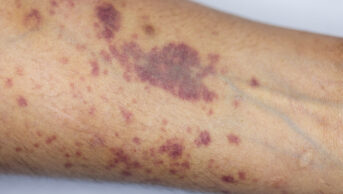
The condition, which is caused by a deficiency of blood clotting factor VIII, causes patients to bleed uncontrollably.
People with haemophilia currently have intravenous (IV) infusions several times per week to manage the condition.
However, Hemlibra (Roche; emicizumab) — which the NHS will fund for around 2,000 patients — is administered as a single subcutaneous injection on either a weekly or fortnightly basis.
The NHS announcement says that this will “dramatically” cut patients’ risk of life-threatening bleeds.
The drug has previously been available on the NHS, but it was restricted to patients who had developed inhibitors — antibodies that prevent factor VIII replacement treatment from working. The new funding widens the scope to treating patients without these inhibitors.
Liz Carroll, chief executive of the Haemophilia Society, said the funding was “fantastic news” as current treatments “can place a significant burden on people with haemophilia and their carers”.
“Today’s decision will mean that people will have the opportunity to have treatment less frequently without IV access, which will enable many to live their lives more freely,” she said.
Simon Stevens, chief executive at the NHS, said the treatment will particularly help children with haemophilia, “reducing treatment time and even ending the dangerous bleeds which can lead to life-threatening cuts and life-changing damage”.