SUSUMU NISHINAGA/SCIENCE PHOTO LIBRARY
Learning objectives
After reading this learning article, you should be able to:
- Recognise the prothrombotic nature of COVID-19 and explain the mechanisms causing increased risk of venous thromboembolism (VTE);
- Understand how to diagnose and assess VTE in suspected/confirmed COVID-19 patients;
- Understand management principles, including the choice and duration of anticoagulation, monitoring and follow up;
- Recognise that concurrent anticoagulation therapy is not a contraindication to COVID-19 vaccination and discuss precautions for COVID-19 vaccine administration in anticoagulated patients.
Since COVID-19 was declared a pandemic in March 2020, there has been extensive human-to-human transmission, resulting in substantial respiratory pathology and manifestations outside the pulmonary parenchyma[1,2]. Initial observations showed that the hyper-inflammatory response induced by COVID-19 often led to systemic coagulation derangement and coagulopathy, with thrombosis an important factor in the pathology and clinical course of infection[3–5].
Observational studies have reported increased incidence of venous thromboembolism (VTE) in hospitalised COVID-19 patients, ranging between 5% to 85%[6–10]. The estimates are dependent on patient characteristics, stage of illness, assessment settings, the amount of anticoagulation/thromboprophylaxis received, and the type of thrombosis reported (i.e. arterial versus venous thrombosis, and whether distal deep vein thromboses [DVTs] were included)[1,11]. Reported thrombotic events were predominantly pulmonary emboli (PEs); presence of thromboembolic complication was associated with more severe disease and increased mortality[12]. However, the true prevalence of VTE in COVID-19 currently remains unknown, owing to the high variability of reported rates in hospitalised patients and a lack of data from the community cohort[13].
This article aims to provide pharmacists with an understanding of VTE to assist the management of COVID-19 patients and will outline the pathophysiology, diagnosis and current guidelines on anticoagulation management of COVID-19 associated thromboembolism.
Aetiology and pathophysiology
Several factors are thought to increase the risk of VTE in COVID-19 infection (Figure 1)[5,14–16].
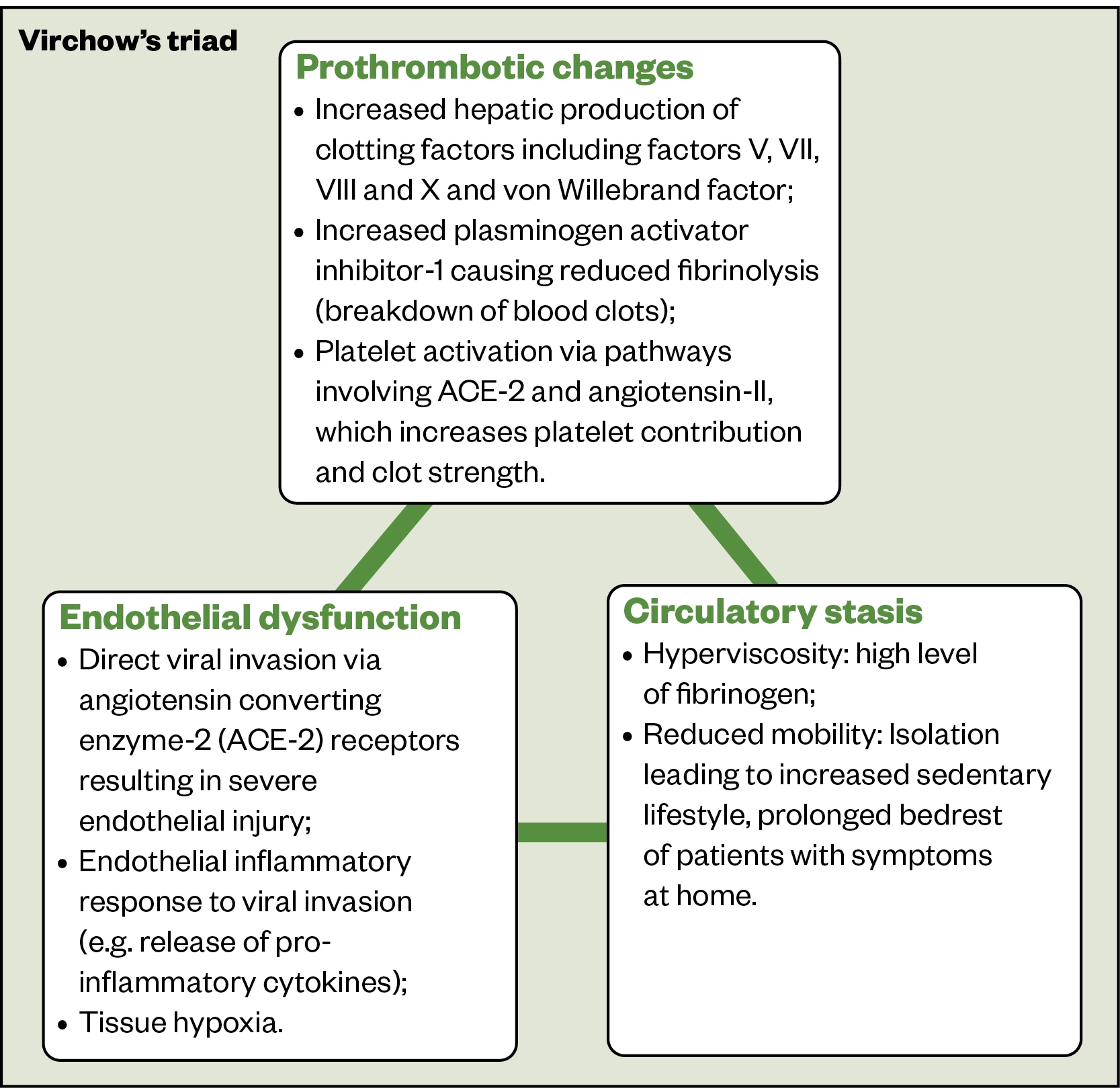
Given such abnormalities involve all three components of Virchow’s triad, COVID-19 patients have an increased risk of thrombosis and VTE. The risks are higher in:
- Hospitalised patients/recent hospital discharges — especially those who are inpatients for ≥3 days and those requiring supplemental oxygen (moderate COVID-19 pneumonia)[17];
- Hospitalised patients requiring ICU admission and/or advanced respiratory support (severe COVID-19 pneumonia) are at highest risk[5,17]. This is owing to excessive inflammatory response in critical illness, use of venous lines, and immobilisation from being sedated, proned or paralysed;
- Women with COVID-19 who are pregnant or have given birth within the past six weeks[17,18]. The risk is increased in females who are hospitalised, and those with ongoing morbidity and limited mobility[18];
- Presence of additional risk factors including: active cancer or therapy; age (over 60 years); comorbidities, such as hypertension, obesity, heart failure and type 2 diabetes mellitus; previous personal or family history of VTE; and use of oestrogen-containing contraception or hormone replacement therapy[13,17,19].
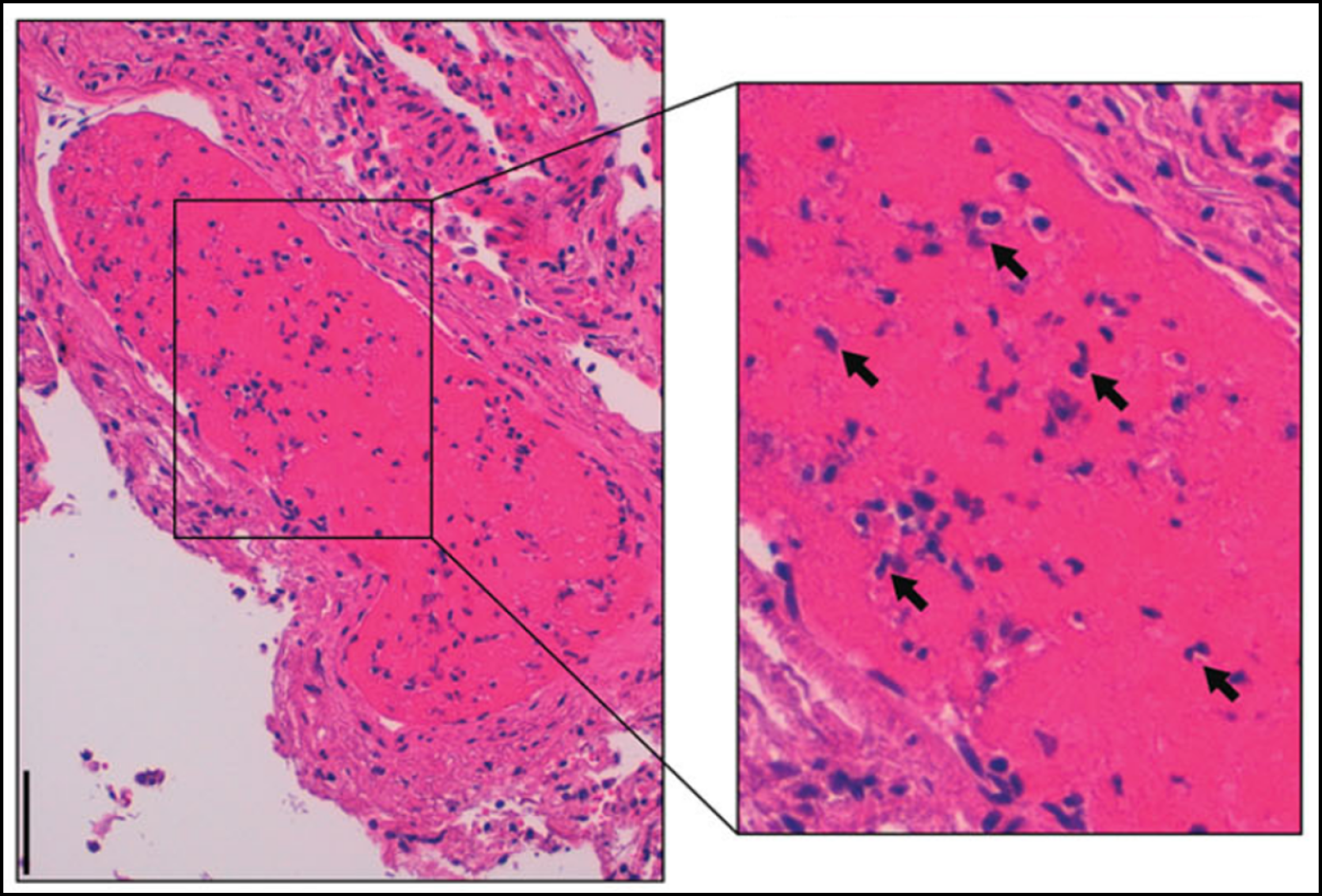
Many COVID-19 patients with PEs have isolated segmental and subsegmental changes (i.e. microthrombi formed within small vessels of the lung secondary to inflammation). These are potentially in situ thrombosis (immunothrombosis; Figure 2[20]), which occurs in all forms of acute respiratory distress syndrome (ARDS), but is more common with COVID-19 infection.
Assessment and diagnosis
Signs and symptoms
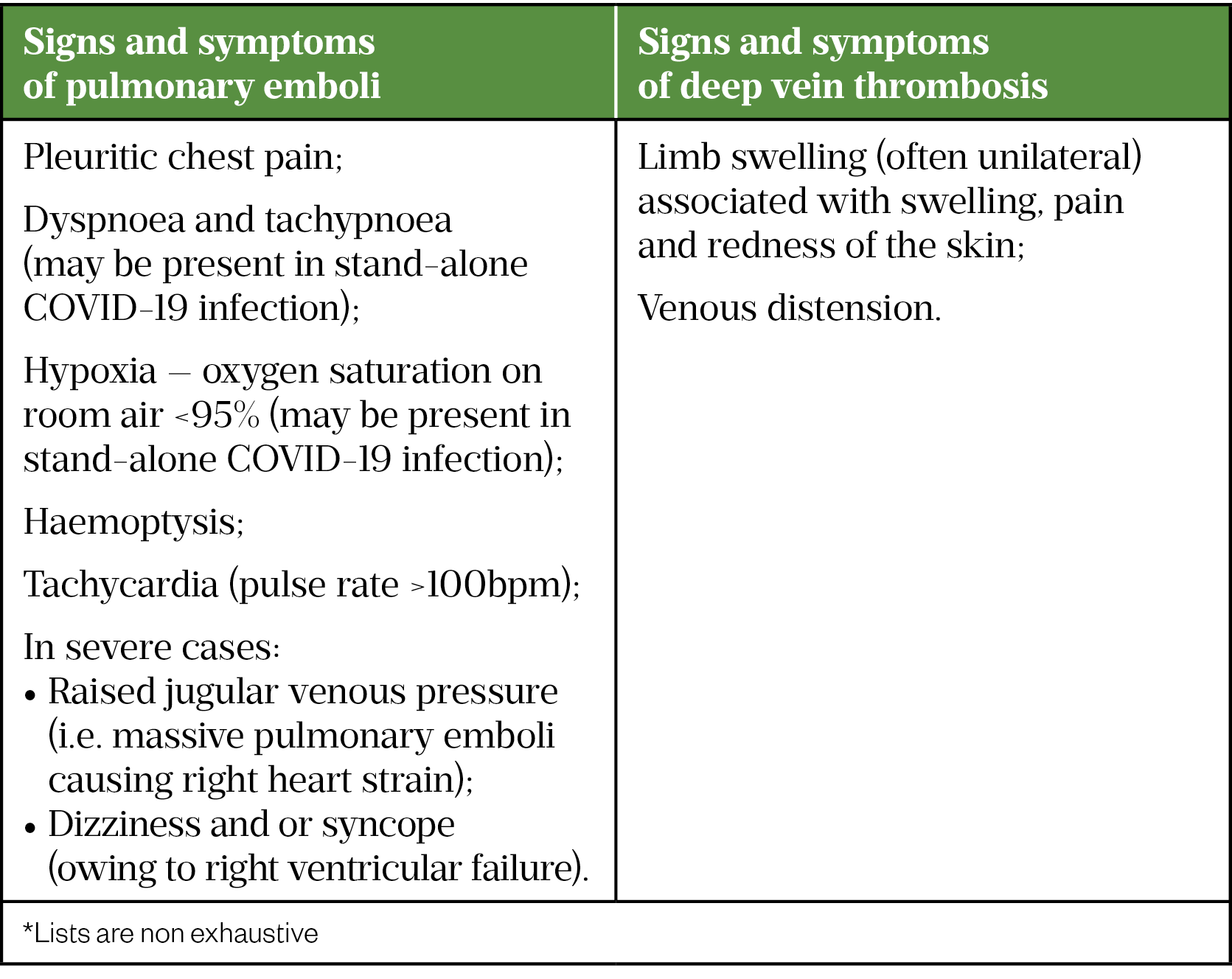
The signs and symptoms of VTE events (PEs and DVTs) associated with COVID-19 infection are similar to conventional VTEs (Table 1)[21,22].
Considering the increased risk of VTE in COVID-19, healthcare professionals should have a low threshold for clinical suspicion of VTE[5]. Community pharmacists may be contacted by patients with suspected/confirmed COVID-19 who have concerns about VTE, and it is important to obtain detailed information, including the patient’s symptoms and past medical history. In the presence of suspected VTE symptoms, especially in high-risk patients, pharmacists should refer the patient for urgent medical assessment.
Diagnosis and investigations
To understand the diagnosis of VTE in COVID-19 patients, it is important to revisit the diagnostic assessment for conventional VTE.
First, a general medical history should be obtained and a physical examination performed[22]. Based on clinical index of suspicion, VTE diagnosis is based on three testing parameters, which are two-level DVT/PE Wells score, quantitative D-dimer test, and imaging studies[22].
Visual summaries of the National Institute for Health and Care Excellence (NICE) recommendations on diagnosis and initial management of suspected DVT and PE can be found here[22].
COVID-19 consideration:
D-dimer testing
D-dimer is the degradation product of crossed-linked fibrin[23]. Although often elevated in patients with acute VTE, D-dimer is non-specific as raised D-dimer may also be observed in active cancer, pregnancy or following trauma and surgery[23]. D-dimer is, therefore, traditionally used as a negative predictive value in diagnosing VTE (i.e. in cases of low clinical suspicion, a negative D-dimer helps exclude diagnosis of thrombosis).
In patients with severe COVID-19 infection, D-dimer is often elevated and is associated with poor outcomes[5,24]. Current guidelines from the British Thoracic Society and the Faculty of Intensive Care Medicine do not support the routine use of D-dimer in isolation to identify VTE and guide decisions on anticoagulation[5,25]. Instead, D-dimer level should be assessed with the overall clinical picture, alongside imaging to confirm diagnosis[5,24,25].
Imaging studies
Performance of imaging studies for VTE diagnosis in COVID-19 patients poses a unique challenge and is more challenging in unstable patients, owing to the risk of transmitting infection to COVID-19 minimised areas and of aerosol transmission to healthcare workers[5,24,25]. Moving and transferring unstable COVID-19 patients is limited owing to high oxygen requirements or invasive ventilation, and renally impaired patients may require continuous renal replacement therapy. Therefore, in critically ill patients, it is important to reliably confirm or exclude PE to avoid unnecessary patient exposure to therapeutic anticoagulation[5,24,25].
When DVT is suspected, Doppler ultrasound scan is recommended as per conventional diagnosis[25]. In unstable patients, a pragmatic approach (e.g. point-of-care bedside ultrasonography) can be combined with standard-of-care objective testing[24].
In COVID-19 patients with sudden deterioration and increasing oxygen requirement disproportionate to the severity of pneumonia, PE should be suspected and confirmed by computed tomography pulmonary angiogram (CTPA), where feasible[5,24,25]. When CTPA is indicated but unable to be performed owing to medical reasons (e.g. renal impairment, contrast allergy, high radiation risk), a scintigraphic perfusion or a ventilation-perfusion (V/Q) lung scan should be considered[25].
In unstable COVID-19 patients, the utility of trans-thoracic echocardiography (TTE) in indirectly diagnosing PE is unclear, as right ventricular (RV) dysfunction is a hallmark of severe disease[5,25]. Elevated troponin, which indicates acute RV myocardial stretch owing to right-sided PE, is often present in severe COVID-19 infection[26]. When RV dysfunction is severe and/or pulmonary hypertension is present, it is important to consider PE diagnosis[5,26,27]. The VTE-BLEED score can be used to identify patients at low risk of bleeding in whom anticoagulation without imaging can be considered and patients at higher risk of bleeding in whom confirmatory imaging is essential[5,28].
Differential diagnosis
Conditions that may present with similar signs and symptoms to DVT include cellulitis, ruptured Baker’s cyst, superficial thrombophlebitis, fracture and calf muscle tear/haematoma following physical trauma[22].
Other causes of symptoms resembling conventional PE include respiratory (pneumothorax, pneumonia, acute chronic lung disease exacerbation), cardiac (acute coronary syndrome), musculoskeletal (chest wall pain), and gastro-oesophageal reflux disease[22]. In addition, the differential diagnosis of VTE in COVID-19 encompasses a wide range of respiratory causes of haemodynamic deterioration (e.g. secondary bacterial infection, ARDS, heart failure and sepsis)[29].
Management
Anticoagulation for acute VTE
Anticoagulants licensed for general acute VTE treatment include direct oral anticoagulants (DOACs), vitamin K antagonists (VKAs), unfractionated heparin (UFH), low molecular weight heparins (LMWHs) and fondaparinux[22]. Fondaparinux is often reserved for patients in whom heparin-based anticoagulants are unsuitable owing to its long half-life and reversibility concerns[24]. Based on clinical and cost-effectiveness evidence, NICE recommends using DOAC as the first-line treatment (with oral-only option more favourable) for the management of VTE in adults[22].
Table 2 outlines the dosage and considerations of DOACs used in VTE treatment[30–34].
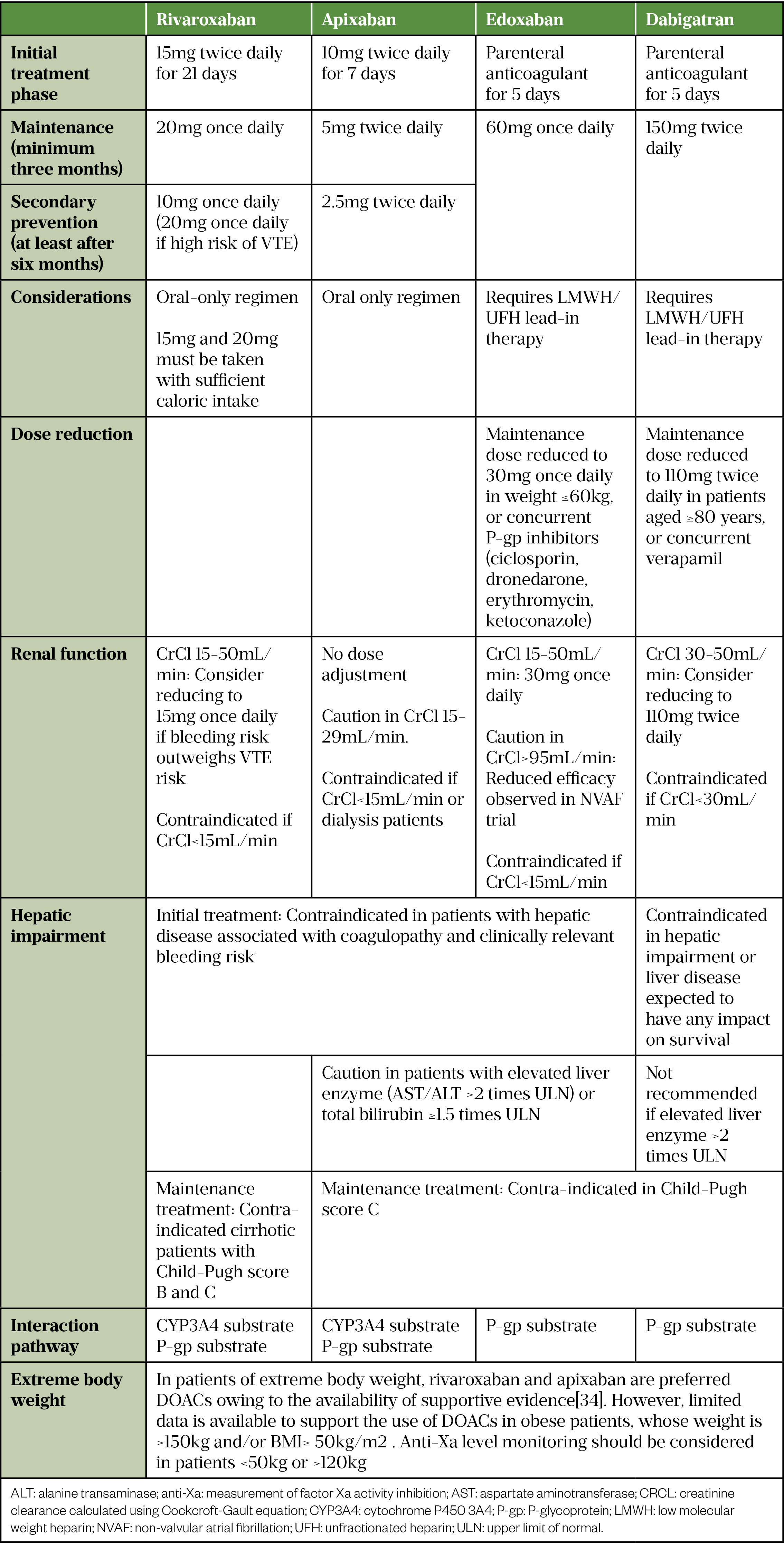
Anticoagulation for thromboembolism in COVID-19
Choice of anticoagulant
Although a DOAC is the first-line treatment for acute VTE, in hospitalised patients with COVID-19-associated thrombosis, parenteral anticoagulation with UFH/LMWH is favoured because:
- Heparin’s polyanionic nature may have anti-inflammatory effects through the neutralisation of pro-inflammatory proteins[35]. Heparin binds to SARS-CoV-2 surface S-glycoprotein (spike) preventing cellular invasion (theoretical benefit)[36];
- Hospitalised patients with COVID-19 are often at high risk of rapid clinical deterioration. Renal impairment (often an acute complication in critically ill patients with COVID-19) can cause reduced excretion of DOACs leading to an increased bleeding risk[24,37,38];
- DOACs are known substrates of CYP3A4 enzyme and/or P-gp transporter (see Table 2) and may interact with antivirals and immunomodulatory therapies[5,24,25,37]. For tocilizumab, the expression of hepatic CYP450 enzyme is suppressed by cytokines (e.g. interleukin-6 [IL-6])[14,39,40]. Suppression of IL-6 by tocilizumab can increase CYP450 activity and reduce plasma exposure of CYP3A4 substrates such as rivaroxaban or apixaban[39]. Owing to its long half-life, the effects on enzyme activity may persist several weeks after stopping therapy[39].
- Critically ill patients may often require invasive monitoring and may undergo invasive procedures at short notice[25]. With shorter half-lives and shorter duration of action, parental anticoagulants are advantageous over oral agents;
- Therapeutic rivaroxaban doses for VTE treatment must be given with sufficient caloric intake. This may be challenging (e.g. in ‘continuous positive airway pressure’ [CPAP] dependent patients)[5].
Among the parenteral anticoagulants, UFH infusion requires frequent coagulation testing; however, in COVID-19 this increases staff exposure and the risk of transmission[37]. LMWH is the preferred parenteral anticoagulant for acute management of VTE in hospitalised COVID-19 patients owing to the availability of standard dosing, ease of administration and less monitoring requirements[24,25,37]. In patients who have a high bleeding risk (e.g. with severe renal impairment or receiving reperfusion therapy in high-risk PE), UFH remains the parenteral anticoagulant of choice during the initial acute phase of VTE treatment[37].
Table 3 outlines the LMWHs licensed in the UK for the treatment of VTE and their dosages.
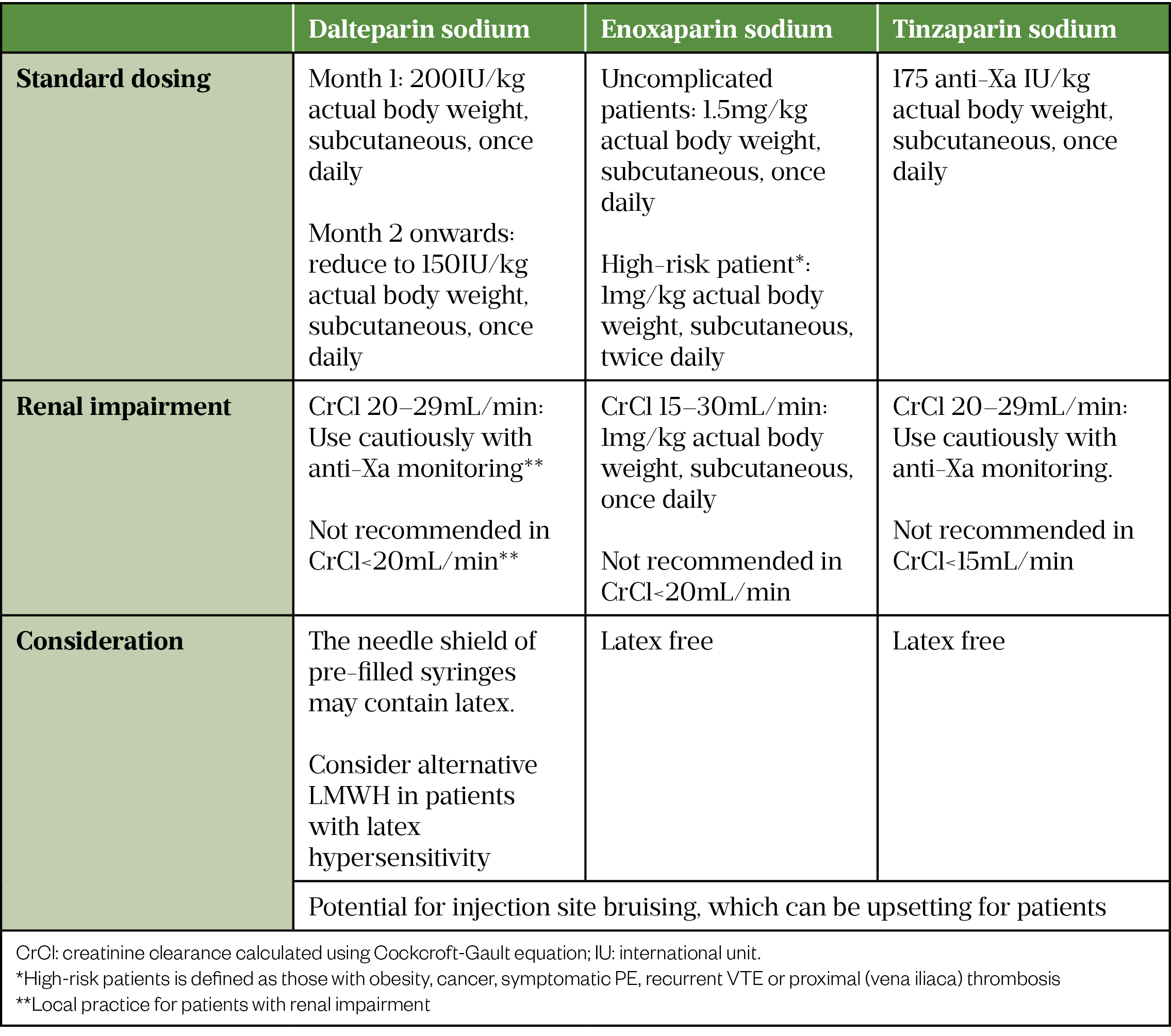
When the patient is no longer clinically compromised and is sufficiently well to take oral medication, anticoagulation switching from LMWH to a DOAC should be considered, provided that no invasive procedures are anticipated and there are no significant drug-drug interactions[5,24,25]. Like general VTE treatment, DOACs provide advantages over VKAs owing to the lack of routine clinic visits for INR monitoring, which minimises patient contact with the healthcare environment and reduces system workload[24,38].
Duration
COVID-19 associated thrombosis is classified as a provoked clotting event, owing to the presence of strong provoking factors outlined in Figure 1. As such, the duration of therapeutic anticoagulation is three months[5,24,25,37]. However, longer durations may be required if chronic thromboembolic pulmonary hypertension or significant chronic thromboembolic disease is suspected[5].
Adverse reaction
As anticoagulants inhibit clotting factor(s) of the coagulation cascade, anticoagulation treatment increases the risk of bleeding. Patients should be counselled on the signs and symptoms of bleeding, which include but are not limited to:
- Tar-coloured stools, blood in urine, prolonged nosebleed lasting more than ten minutes, bleeding of gums or from cuts that take a long time to stop;
- Bruising or bleeding under the skin with swelling or discomfort;
- Headache, dizziness, tiredness, paleness or weakness;
- Coughing up blood or vomiting blood or material that looks like coffee grounds;
- Loss of consciousness or drowsiness.
Patients should be reassured that the benefits of anticoagulation for VTE treatment outweigh the risk of bleeding and should be given details of who to contact (both in and out of hours) in the event of bleeding. Patients should be advised that uncontrollable bleeding requires immediate medical attention and no further doses should be taken until this is reviewed.
LMWH can induce hyperkalaemia owing to its inhibitory effect on aldosterone synthesis, although this arises within a few days of initiation, it is often transient[41]. Plasma potassium levels should be monitored during the acute phase of treatment.
Serious skin reactions, including Stevens-Johnson syndrome/toxic epidermal necrolysis and DRESS syndrome, have been reported in the first week of rivaroxaban use, and should therefore be discontinued and the patient switched to an alternative anticoagulant at the first appearance of a severe skin rash, or any other sign of mucosal hypersensitivity[30].
Lifestyle management
Patients who have experienced VTE episode(s) are at a higher risk of recurrence[22]. The World Health Organization (WHO) recommends that all adults 18–64 years of age should participate in at least 150 minutes of moderate-intensity aerobic physical activity (e.g. walking, running, dancing, hiking), or at least 75 minutes of vigorous-intensity aerobic physical activity, throughout the week[42]. Individuals with COVID-19 and/or VTE diagnosis should follow such guidance, as inactivity/immobilisation over prolonged periods will further increase the risk of VTE.
Eating home-cooked meals, fruits and vegetables, monitoring portion sizes, and limiting sugar, salt and alcohol intake (which may increase the risk of bleeding), are important dietary recommendations[43]. Although green vegetables are highly recommended, patients on warfarin should keep consistency in vitamin K-rich food consumption (e.g. leafy green vegetables) to ensure they do not reverse the anticoagulation effect. Patients on rivaroxaban (15 mg and 20 mg doses) should ensure the DOAC is taken with high-calorie meals[44].
Anticoagulation and COVID-19 vaccination
Public Health England guidance states that COVID-19 vaccines can be administered intramuscularly to individuals on stable anticoagulation therapy, including stabilised patients on a DOAC or those on warfarin who are up to date with their scheduled INR testing and with the latest INR below the upper level of the therapeutic range[45]. A fine needle (equal to 23 gauge or finer) should be used for the vaccination, followed by firm pressure applied to the site without rubbing for at least two minutes[44]. The individual/carer should be informed about the risk of haematoma[45].
Monitoring and follow-up
Prior to starting anticoagulation treatment, a baseline clotting screen, full blood count, renal and liver function test should be performed[22]. Owing to the risk of heparin-induced thrombocytopenia (HIT), patients who are anticoagulated with UFH/LMWH (i.e. hospitalised COVID-19 patients with VTE) should have platelet count measured before initiation of anticoagulation[46]. Thrombocytopenia is defined as a platelet count less than 150×109/L; HIT should be considered if the platelet count falls by 30% or more between day 4 and 14 of heparin administration[46].
Critically ill patients, those with impaired renal function or extreme body weight, or who are anticoagulated with LMWH should have their anti-Xa level monitored after the third day of treatment to ensure appropriate dosing[47]. To accurately measure the peak anti-Xa level, the specimen should be collected 3 to 4 hours post injection[47]. When the level is in range (0.5–1.0 iu/mL for twice-daily dosing and 1.0–1.5 iu/mL for once-daily dosing), repeated anti-Xa is not routinely required unless clinically indicated (e.g. deteriorating renal function or increased bleeding risks)[47]. Hospital pharmacists can identify patients who will need anti-Xa level checking, ensure accurate sample timing and assist with interpreting level results if appropriate.
Prior to discharge, patients should be provided with information on VTE (e.g. signs of recurrence, any additional complications, and anticoagulation therapy). Patients who are discharged on LMWH should receive self-injection training if appropriate and should be supplied with sharp bins to ensure safe disposal.
The British Thoracic Society recommends that patients should have a formal review (telephone/face-to-face) during the first week after discharge to ensure therapeutic compliance[48]. It is important to assess any worsening symptoms, reinforce the importance of regular dosing schedule, and enquire about the presence of any adverse effects (e.g. bleeding)[49]. The follow-up review also provides an opportunity to perform any outstanding investigations/screening. In the context of the COVID-19 pandemic, remote consultations should be the preferred method of follow-up, with in-person visits reserved for scenarios that cannot be addressed remotely or may potentially require hospitalisation[50].
As previously mentioned, patients with COVID-19 related thromboembolism are often anticoagulated for at least three months. In renally impaired patients treated with a DOAC, the European Heart Rhythm Association suggests that if the CrCl is <60mL/minute, the frequency of monitoring (in months) can be guided by the CrCl divided by ten (e.g. if CrCl is 30mL/min then the renal function [and the prescribed dose] should be reassessed every three months)[38]. Pharmacists working in primary care may adopt this practice; however, it should be noted that this recommendation was made based on DOAC use in NVAF.
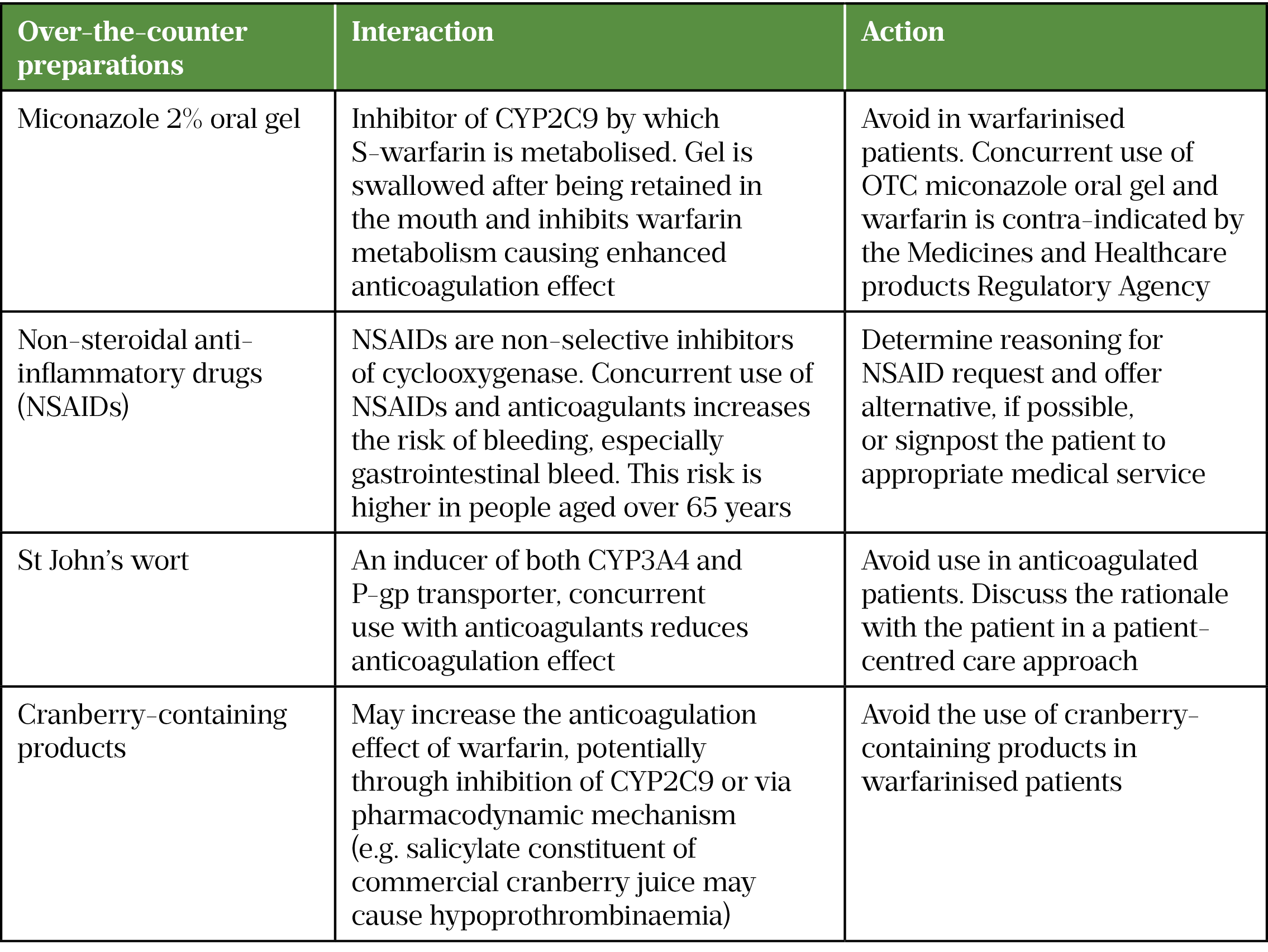
Given that anticoagulation is a high-risk therapy that can potentially cause harm and hospital admission, community pharmacists can support patients by conducting the new medicines service and providing advice on potential drug interactions with over-the-counter medicines (see Table 4[38,51,52]).
Best practice recommendations
Pharmacists can contribute to the management of VTE in COVID-19 patients by:
- Advising other healthcare professionals and patients on the increased risk of VTE in COVID-19 infection. Ensuring VTE risk assessment is performed and thromboprophylaxis is prescribed in accordance with national/local guidance — this may be upon hospital admission or in a hospital-led acute care community unit;
- Recognising the potential signs and symptoms of VTE. Pharmacists working in primary care can recognise and signpost isolated COVID-19 patients with suspected symptoms for further urgent investigation as appropriate;
- Pharmacists working in partnership with the thrombosis multidisciplinary team (MDT) can get involved in the development of treatment pathway, including provision of specialist advice on drug interactions and anticoagulation choice and any monitoring requirements for specific patient populations.
Secondary care pharmacists can contribute to the provision of patient-centred care through liaison with the MDT and discussion with the patient to form patient-specific treatment plans, taking into account both the patient’s preference and their clinical circumstance.
Useful resources
Acknowledgement
The author would like to thank Jannat Muen, anticoagulation and thrombosis prevention pharmacist, and Joshua Saitch, acute care common stem CT2 trainee in intensive care medicine, both at Sheffield Teaching Hospitals NHS Foundation Trust, for their valuable comments and review of this article.
- 1Jiménez D, García-Sanchez A, Rali P, et al. Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019. Chest. 2021;159:1182–96. doi:10.1016/j.chest.2020.11.005
- 2Guidance: COVID-19: epidemiology, virology and clinical features. Public Health England. 2021.https://www.gov.uk/government/publications/wuhan-novel-coronavirus-background-information/wuhan-novel-coronavirus-epidemiology-virology-and-clinical-features (accessed Jan 2022).
- 3Oran DP, Topol EJ. Prevalence of Asymptomatic SARS-CoV-2 Infection. Annals of Internal Medicine. 2020;173:362–7. doi:10.7326/m20-3012
- 4Hunt BJ, De Paula EV, McLintock C, et al. Prophylactic anticoagulation for patients in hospital with covid-19. BMJ. 2021;:n487. doi:10.1136/bmj.n487
- 5BTS Guidance on Venous Thromboembolic Disease in patients with COVID-19. British Thoracic Society. 2021.https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/bts-guidance-on-venous-thromboembolic-disease-in-patients-with-covid-19/ (accessed Jan 2022).
- 6Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020;191:145–7. doi:10.1016/j.thromres.2020.04.013
- 7Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi:10.1182/blood.2020006520
- 8Middeldorp S, Coppens M, Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18:1995–2002. doi:10.1111/jth.14888
- 9Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–4. doi:10.1111/jth.14830
- 10Helms J, Tacquard C, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–98. doi:10.1007/s00134-020-06062-x
- 11Price LC, McCabe C, Garfield B, et al. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J. 2020;56:2001608. doi:10.1183/13993003.01608-2020
- 12Loo J, Spittle DA, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76:412–20. doi:10.1136/thoraxjnl-2020-216243
- 13COVID-19 and VTE/Anticoagulation: Frequently Asked Questions. American Society of Hematology. 2021.https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation (accessed Jan 2022).
- 14Ahmed S, Zimba O, Gasparyan AY. Thrombosis in Coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin Rheumatol. 2020;39:2529–43. doi:10.1007/s10067-020-05275-1
- 15Voicu S, Ketfi C, Stépanian A, et al. Pathophysiological Processes Underlying the High Prevalence of Deep Vein Thrombosis in Critically Ill COVID-19 Patients. Front. Physiol. 2021;11. doi:10.3389/fphys.2020.608788
- 16Mehta JL, Calcaterra G, Bassareo PP. COVID ‐19, thromboembolic risk, and Virchow’s triad: Lesson from the past. Clin Cardiol. 2020;43:1362–7. doi:10.1002/clc.23460
- 17COVID-19 Rapid Guideline: Managing COVID-19. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ng191 (accessed Jan 2022).
- 18Coronavirus (COVID-19) infection and pregnancy. Royal College of Obstetricians and Gynaecologists. 2021.https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/ (accessed Jan 2022).
- 19Anderson FA Jr, Spencer FA. Risk Factors for Venous Thromboembolism. Circulation. 2003;107. doi:10.1161/01.cir.0000078469.07362.e6
- 20Nicolai L, Leunig A, Brambs S, et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020;142:1176–89. doi:10.1161/circulationaha.120.048488
- 21Freund Y, Rousseau A, Guyot-Rousseau F, et al. PERC rule to exclude the diagnosis of pulmonary embolism in emergency low-risk patients: study protocol for the PROPER randomized controlled study. Trials. 2015;16. doi:10.1186/s13063-015-1049-7
- 22Venous thromboembolic diseases: diagnosis, management and thrombophilia testing, NICE guideline [NG158]. National Institute for Health and Care Excellence. 2020.https://www.nice.org.uk/guidance/ng158 (accessed Jan 2022).
- 23Siegal D, Lim W. Venous Thromboembolism. Hematology. 2018;:2102–12. doi:10.1016/b978-0-323-35762-3.00142-6
- 24Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18:1859–65. doi:10.1111/jth.14929
- 25Clinical guide for the prevention, detection and management of thromboembolic disease in patients with COVID-19. Intensive Care Medicine Anaesthesia COVID-19. 2020.https://icmanaesthesiacovid-19.org/clinical-guide-prevention-detection-and-management-of-vte-in-patients-with-covid-19 (accessed Jan 2022).
- 26Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. Journal of the American College of Cardiology. 2020;75:2352–71. doi:10.1016/j.jacc.2020.03.031
- 27ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. European Society of Cardiology. 2020.https://www.escardio.org/static-file/Escardio/Education-General/Topic%20pages/Covid-19/ESC%20Guidance%20Document/ESC-Guidance-COVID-19-Pandemic.pdf (accessed Jan 2022).
- 28Klok FA, Barco S, Konstantinides SV. External validation of the VTE-BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thromb Haemost. 2017;117:1164–70. doi:10.1160/th16-10-0810
- 29Tal S, Spectre G, Kornowski R, et al. Venous Thromboembolism Complicated with COVID-19: What Do We Know So Far? Acta Haematol. 2020;143:417–24. doi:10.1159/000508233
- 30Xarelto 20mg film-coated tablets. Electronic Medicines Compendium. 2019.https://www.medicines.org.uk/emc/product/2793 (accessed Jan 2022).
- 31Eliquis 5 mg film-coated tablets . Electronic Medicines Compendium. 2020.https://www.medicines.org.uk/emc/product/2878 (accessed Jan 2022).
- 32Lixiana 60mg Film-Coated Tablets . Electronic Medicines Compendium. 2020.https://www.medicines.org.uk/emc/product/6905 (accessed Jan 2022).
- 33Pradaxa 150 mg hard capsules . Electronic Medicines Compendium. 2020.https://www.medicines.org.uk/emc/product/4703 (accessed Jan 2022).
- 34Martin KA, Beyer‐Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J. Thromb. Haemost. 2021;19:1874–82. doi:10.1111/jth.15358
- 35Gozzo L, Viale P, Longo L, et al. The Potential Role of Heparin in Patients With COVID-19: Beyond the Anticoagulant Effect. A Review. Front. Pharmacol. 2020;11. doi:10.3389/fphar.2020.01307
- 36Mycroft-West CJ, Su D, Pagani I, et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb Haemost. 2020;120:1700–15. doi:10.1055/s-0040-1721319
- 37Moores LK, Tritschler T, Brosnahan S, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019. Chest. 2020;158:1143–63. doi:10.1016/j.chest.2020.05.559
- 38Steffel J, Collins R, Antz M, et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Europace. 2021;23:1612–76. doi:10.1093/europace/euab065
- 39RoActemra 20mg/ml Concentrate for Solution for Infusion. Electronic Medicines Compendium. 2021.https://www.medicines.org.uk/EMC/medicine/22311 (accessed Jan 2022).
- 40Goralski K, Ladda M, McNeil J. Drug-Cytokine Interactions. In: Pai M, Kiser J, Gubbins P, et al., eds. Drug Interactions in Infectious Diseases: Mechanisms and Models of Drug Interactions. Cham, Switzerland: : Humana Press 2018.
- 41Thomas CM, Thomas J, Smeeton F, et al. Heparin-induced hyperkalemia. Diabetes Research and Clinical Practice. 2008;80:e7–8. doi:10.1016/j.diabres.2008.01.019
- 42World Health Organization. Stay physically active during self-quarantine. 2020.https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/publications-and-technical-guidance/noncommunicable-diseases/stay-physically-active-during-self-quarantine (accessed Jan 2022).
- 43Food and Nutrition tips during self-quarantine. World Health Organization. 2020.http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/technical-guidance/food-and-nutrition-tips-during-self-quarantine (accessed Jan 2022).
- 44Rivaroxaban (Xarelto▼): reminder that 15 mg and 20 mg tablets should be taken with food. gov.uk. 2019.https://www.gov.uk/drug-safety-update/rivaroxaban-xarelto-reminder-that-15-mg-and-20-mg-tablets-should-be-taken-with-food (accessed Jan 2022).
- 45COVID-19 SARS-CoV-2. In: Immunisation against infectious disease – The Green Book. London: : Public Health England 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043861/Greenbook-chapter-14a-24Dec21.pdf (accessed Jan 2022).
- 46Howard LSGE, Barden S, Condliffe R, et al. British Thoracic Society Guideline for the initial outpatient management of pulmonary embolism (PE). Thorax. 2018;73:ii1–29. doi:10.1136/thoraxjnl-2018-211539
- 47Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;:n/a-n/a. doi:10.1111/bjh.12059
- 48Garcia DA, Baglin TP, Weitz JI, et al. Parenteral Anticoagulants. Chest. 2012;141:e24S-e43S. doi:10.1378/chest.11-2291
- 49Anticoagulants and monitoring. Anticoagulation UK. https://www.anticoagulationuk.org/provision/anticoagulants-and-monitoring (accessed Jan 2022).
- 50Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up. Journal of the American College of Cardiology. 2020;75:2950–73. doi:10.1016/j.jacc.2020.04.031
- 51Stockley’s Drug Interactions. Medicines Complete. https://about.medicinescomplete.com/publication/stockleys-drug-interactions/ (accessed Jan 2022).
- 52Miconazole (Daktarin): over-the-counter oral gel contraindicated in patients taking warfarin. gov.uk. 2017.https://www.gov.uk/drug-safety-update/miconazole-daktarin-over-the-counter-oral-gel-contraindicated-in-patients-taking-warfarin (accessed Jan 2022).
1 comment
You must be logged in to post a comment.

Interesting article