Wikimedia Commons
The National Institute for Health and Care Excellence (NICE) has asked pharmaceutical company, Merck, to submit a Cancer Drugs Fund (CDF) proposal for its immunotherapy drug avelumab (Bavencio; Merck).
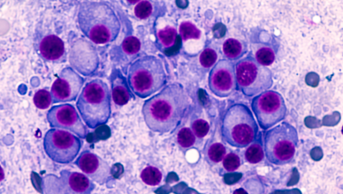
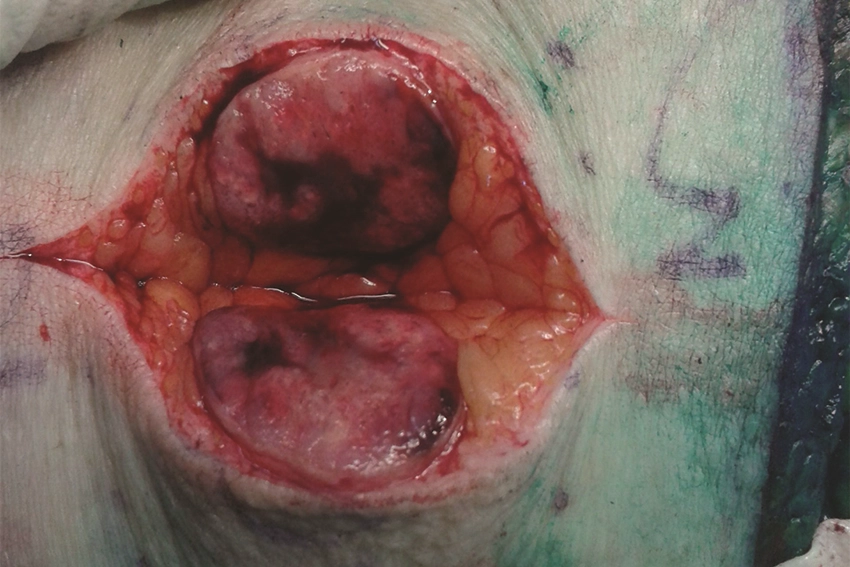
The drug was assessed by NICE as a treatment for people with a rare and painful type of skin cancer called Merkel cell carcinoma, in which tumours form close to nerve endings in the top layer of the skin.
The NICE committee found that current clinical data for avelumab suggest the drug can extend patient survival compared with chemotherapy. But evidence is limited and data are still being collected meaning that final conclusions about its overall efficacy cannot yet be drawn.
If submitted to the CDF, patients will have access to the drug while the company gathers more data. This additional data will then be submitted to the NICE committee for it to re-consider whether avelumab should be made routinely available on the NHS.
“Merkel cell carcinoma is a rare and aggressive form of cancer,” said Carole Longson, director of the centre for health technology evaluation at NICE.
“There are limited treatment options and the cancer can spread rapidly, which we know can be frightening for both patients and families.
“Avelumab is a promising treatment that has the potential to be cost effective. I hope Merck will work with us to submit a CDF proposal,” she added.
According to NICE, avelumab works by harnessing the power of the patient’s own immune system to destroy their cancerous cells.