Dr P Marazzi / Science Photo Library
Patients with type 1 diabetes could be given the choice of using blood glucose monitoring devices rather than standard capillary blood glucose monitoring, under draft guidelines from the National Institute for Health and Care Excellence (NICE).
The draft guidelines, published on 24 November 2021, said the update was “likely to result in broader access” to intermittently scanned continuous glucose monitoring (isCGM) and real-time continuous glucose monitoring (CGM) devices.
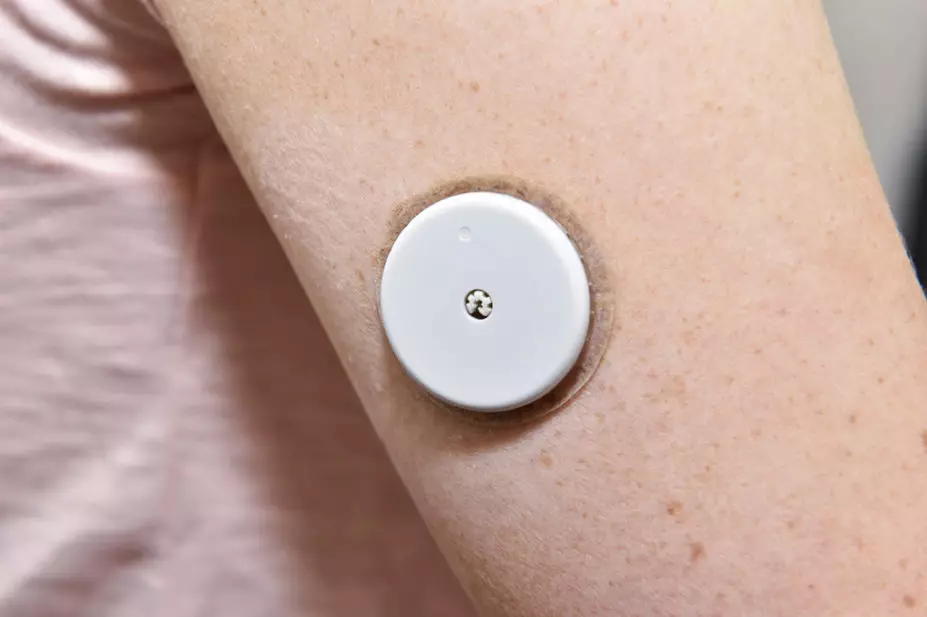
Both devices have a sensor that sits under the skin on a patients’ arm, either monitoring blood glucose levels continuously or intermittently when scanned — also known as ‘flash’ monitoring.
The guidelines were published as NHS England said on 25 November 2021 that around 125,000 people, or half of eligible patients, with type 1 diabetes in England were using flash glucose monitors as of July 2021.
Under current guidance, clinicians are advised against routinely offering CGM to adults with type 1 diabetes, except where patients meet a set of five criteria, including “more than one episode a year of severe hypoglycaemia with no obvious preventable cause”.
However, the draft guidance advises offering adults with type 1 diabetes “a choice of real-time CGM or isCGM”.
“If a person is unable or does not wish to use any real-time CGM or isCGM device, offer capillary blood glucose monitoring,” it adds.
In the draft guidance, the committee said that it had found “enough evidence in key outcomes, such as HbA1c, time in range and severe or nocturnal hypoglycaemia, to demonstrate that both real-time CGM and intermittently scanned CGM (isCGM, or ‘flash’) provide clinical benefits over standard self-monitoring of blood glucose”.
In April 2019, NHS England announced that it would fund flash monitors for 20% of eligible patients through clinical commissioning group budgets until March 2021.
However, in a press release published on 25 November 2021, NHS England said it had “significantly exceeded” this, with more than 45% of eligible patients benefiting from the monitors in March 2021 and around 125,000, or half, using the monitors by July 2021.
Amanda Pritchard, chief executive of NHS England, said: “Flash glucose monitoring is a great example of where technology and digital solutions can help individuals to live more independent lives, better manage their own conditions, and avoid more acute health problems developing.”
Chris Askew, chief executive at Diabetes UK, said the technology had the power to “transform” the lives of those who use it, improving quality of life and diabetes self-management.
“We also know that, for those who do have access to flash monitors, it has been particularly beneficial while face-to-face diabetes care has been understandably limited during the restrictions of the COVID-19 pandemic,” he added.
Hannah Beba, a consultant pharmacist specialising in diabetes at NHS Leeds Clinical Commissioning Group, said that the expansion of access set out by NICE around technology in the management of type 1 and type 2 diabetes was “borne out of a plethora of compelling evidence”.
“Access to technology is a fundamental part of achieving parity of esteem for people living with diabetes,” she added.
“This document is welcomed by the whole diabetes community, both healthcare professionals and people living with diabetes alike.”
Beba said that healthcare was now moving from an era where technology was a “niche” area of practice based in secondary care to one where technology now needs to be understood and used appropriately across all healthcare systems.
“With this follows complexity in training, information governance and consideration must be given to digital poverty,” she continued.
The guidance is out for consultation until 22 December 2021 and the final guidelines are expected to be published on 31 March 2022.
Read more: Diabetes in hospital — could more specialist pharmacists reduce high error rates?