Panther Media GmbH / Alamy Stock Photo
The National Institute for Health and Care Excellence (NICE) has recommended the use of real-time continuous glucose monitoring for adults and children living with type 1 diabetes for the first time.
The updated NICE guideline for type 1 diabetes in adults, published on 31 March 2022, also expands on recommendations to use intermittently scanned glucose monitoring — also known as ‘flash monitoring’ — to all patients with type 1 diabetes.
The guidelines note that these recommendations mean that patients will now have a choice in picking the technology that is right for them “based on their individual preferences, needs, characteristics, and the functionality of the devices available”.
Under previous guidance, clinicians were advised against routinely offering continuous glucose monitoring to adults with type 1 diabetes, except where patients met a set of five criteria, including “more than one episode a year of severe hypoglycaemia with no obvious preventable cause”.
However, the new guidance advises offering adults with type 1 diabetes “choice of real-time continuous glucose monitoring or intermittently scanned continuous glucose monitoring”.
“If a person cannot use or does not want real-time continuous glucose monitoring or intermittently scanned continuous glucose monitoring, offer capillary blood glucose monitoring,” it adds.
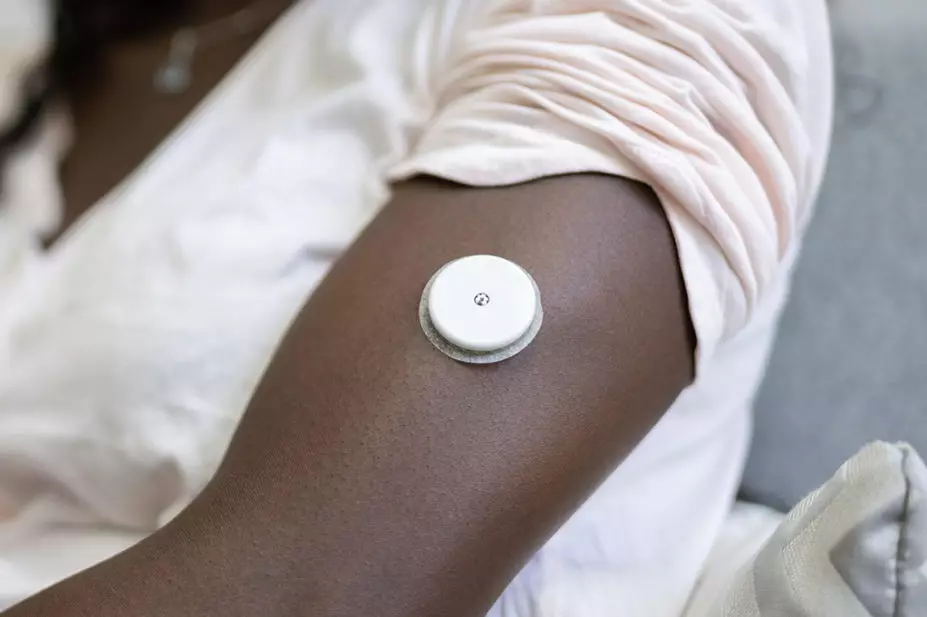
Both devices used for real-time continuous glucose monitoring and intermittently scanned continuous glucose monitoring have a sensor that sits under the skin on a patient’s arm . The monitors link to an app on the patient’s phone, and unlike conventional monitors, allow patients to view patterns over time.
In addition to updating its type 1 diabetes guidance, on 31 March 2022 NICE also updated its type 2 diabetes guidance to recommend extending the use of flash monitoring to adults with type 2 diabetes on insulin therapy.
Paul Chrisp, director of the centre for guidelines at NICE, said: “By recommending the use of either real-time or flash monitoring, our independent committee has made recommendations that will be a step forward in helping all people with type 1 diabetes manage their condition.
“Many people find finger-prick testing to be painful and time consuming and the introduction of technology for all people living with type 1 diabetes will reduce this considerably.
“This group of people also live with the constant worry of suffering from an attack brought on by dangerously low blood sugar while they sleep. Having an alarm which will alert them if this happens will give them the peace of mind knowing they will wake up in the morning.”
Chris Askew, chief executive at Diabetes UK, said the “landmark” guidelines would be “transformational” for people living with diabetes.
“Having campaigned for many years for wider access to flash and continuous glucose monitoring, and contributed to NICE’s consultation, we are delighted that the voice of people with diabetes has been heard, and that our calls have been listened to,” he said.
“What we are seeing today is a key shift in thinking — a move to recognising that technology is an integral part of diabetes management, not simply an added luxury.”
Philip Newland-Jones, consultant pharmacist specialising in diabetes and endocrinology at University Hospital Southampton NHS Foundation Trust, said that the change in national guidance would be “life-changing” for some individuals.
“When we consider NICE now states that either [device] should be offered in preference to finger prick testing in type 1 diabetes, this is a huge change in the management of type 1 diabetes.”
Newland-Jones also said the results of the FLASH-UK trial, presented at the Diabetes UK conference on 30 March 2022, showed that in those switched to intermittently scanned continuous glucose monitoring, average HbA1c improved over 24 weeks from 8.7% (72mmol/mol) to 7.9% (63mmol/mol) with two extra hours per day spent within target range, and 80% less hypoglycaemia.
“The cost per QALY [quality adjusted life year] gained in this particular randomised controlled trial was estimated at £5,191, which shows that this intervention is extremely cost effective for the NHS (the target being <£20,000 in most circumstances),” he said.
“For people with type 2 diabetes, who previously wouldn’t have been able to have access to intermittently scanned continuous glucose monitoring, opening access to those on multiple daily insulin injections meeting certain criteria, or those on insulin who require third party assistance for monitoring is likely to significantly improve the quality of life of those with diabetes and their families.”
According to a press release published by NHS England on 31 March 2022, the ‘NHS long-term plan’ included a target to ensure 20% of people with type 1 diabetes were benefiting from flash monitors by March 2021, but recent data show that the NHS “significantly exceeded” that goal, with nearly three fifths already accessing the technology.


