MHRA
A 45-minute e-learning module targeted at improving the reporting of suspected adverse drug reactions (ADRs) has gained approval from the European Accreditation Council for Continuing Medical Education (EACCME).
Making medications safe relies on doctors and patients reporting ADRs, but according to the Medicines and Healthcare products Regulatory Agency (MHRA), there are issues with under-reporting.
A survey from the Strengthening Collaboration for Operating Pharmacovigilance in Europe (SCOPE) Joint Action project, which created the new module, found that many countries lacked sustainable educational resources on ADR reporting.
The new module will see doctors awarded one EACCME credit on completion, a spokesperson from the MHRA told The Pharmaceutical Journal. This is equivalent to one hour’s continuing professional development (CPD) or continuing medical education (CME).
MHRA, which regulates medicines and medical devices in the UK, is leading the SCOPE Joint Action Project.
A spokesperon said: “Being vigilant not only in the prompt identification and reporting of suspected ADRs is important, but so is staying up-to-date with the latest safety information.”
The module follows on from an EU-wide ADR awareness raising week campaign that took place in November 2016, the spokesperson noted, which involved 21 medicine regulators across the EU. Regulators from Croatia, Ireland, Italy, Latvia, Lithuania and Romania have already encouraged use of the module, the spokesperson said.
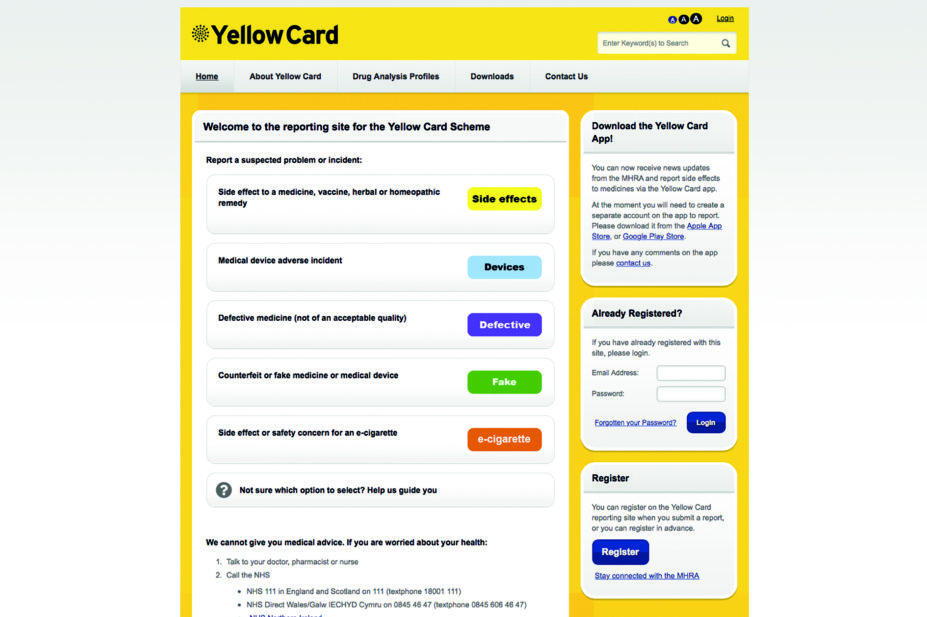
Doctors in the UK can report ADRs online on the MHRA’s Yellow Card page and through clinical software systems such as systmOne and VISION. According to the MHRA spokesperson, ADR reports are increasing annually: 2016 saw 42,000 suspected ADR reports via the Yellow Card scheme, which is the highest to date, and the database now holds more than 820,000 records.
“We’ve created this e-learning module to help doctors so they can have confidence that their reports are making a difference,” said Mick Foy, group manager of the MHRA’s vigilance and risk management of medicines division in a statement published on 16 June 2017. “Doctors are critical to this as their position on the front line of care means they are often the first to recognise an adverse drug reaction.”