Annette Lozinski / Alamy Stock Photo
Healthcare safety investigators were unable to conclude whether the scheduling of morphine sulfate oral solution contributed to the death of a patient, partly because they could not contact the patient’s community pharmacy.
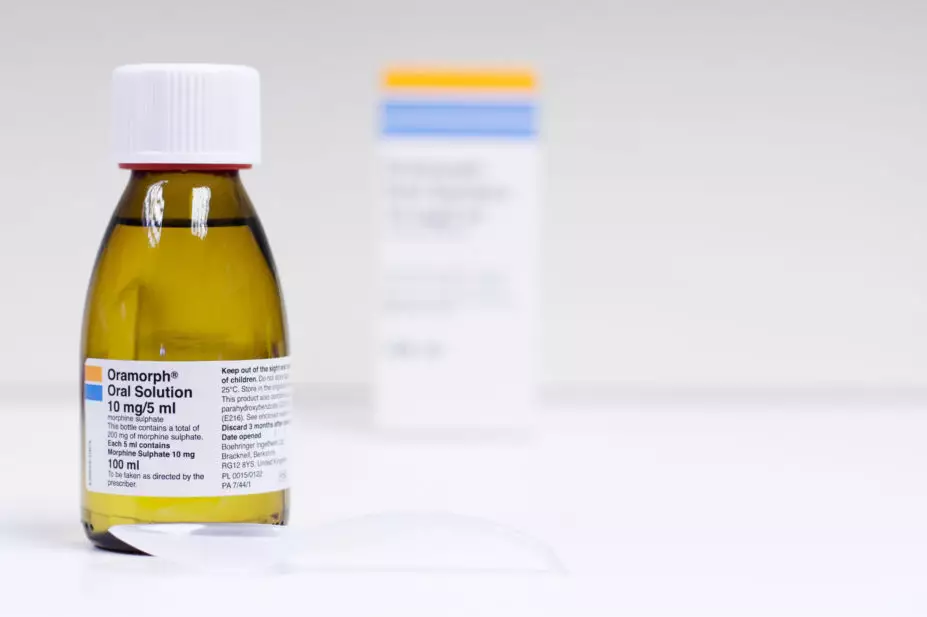
A report, published by the Healthcare Safety Investigation Branch (HSIB) on 28 April 2022, detailed an investigation into the death of a patient, aged 89 years, following an overdose of morphine sulfate oral solution that his GP had prescribed after he fell at his home.
According to the investigation, the GP had prescribed a dose of 1.25–2.5mL to be taken up to every 4 hours when required.
However, the patient and his wife understood the ‘10mg in 5mL’ strength on the morphine bottle’s label to be the dose and the patient took three doses of 5mL — two to four times the suggested dose — over the course of one day. The patient later died from the overdose.
Investigators looked into 11 lines of enquiry relating to the case, including the “implication of oral morphine solution 10mg in 5ml being a Schedule 5 controlled drug, rather than the tighter controls of a Schedule 2 for other forms of morphine”.
The HSIB report cited an investigation carried out by The Pharmaceutical Journal, which revealed that oral morphine sulfate solution has been directly linked to the cause of death in 13 ‘prevention of future death reports’ issued by coroners since 2013 and highlighted calls for the drug to be rescheduled.
In the outcome of this line of enquiry, the HSIB investigators said these concerns relate “to large quantities of oral morphine being provided for chronic use”, which it said was not relevant to the case.
However, they added that “the investigation was unable to engage with the community pharmacy to understand any impact” the scheduling may have had on the patient’s care.
As a result of being unable to contact the community pharmacy that supplied the medicine to the patient, the HSIB recommended “professional bodies and regulators highlight the importance to their membership of participating in HSIB safety investigations to ensure that all relevant leaning can be obtained”.