Shutterstock.com
Community pharmacy teams are currently required to supply wholesalers with prescription details before orders of two varicella vaccine brands are approved, owing to supply problems.
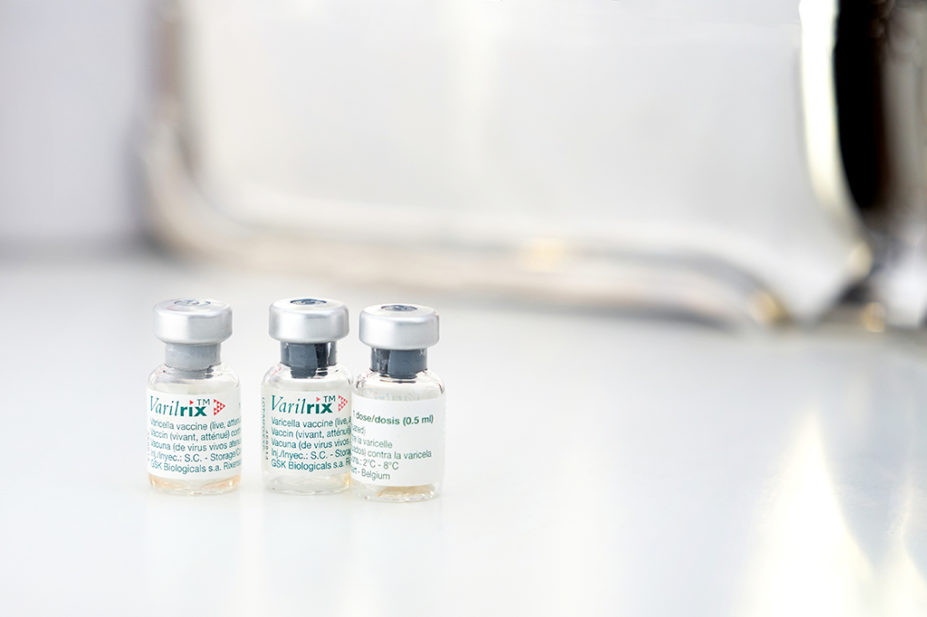
A medicine supply notification, issued by the Department of Health and Social Care on 12 August 2022, said limited supplies of Varivax (MSD) and Varilrix (GlaxoSmithKline) injections — provided to at-risk patients to protect against the varicella zoster virus that causes chickenpox — are available “until mid-October 2022”.
As a result, wholesaler AAH Pharmaceuticals has implemented ordering quotas for Varilrix “alongside prescription validation for primary care, to ensure equitable and appropriate distribution during this period”.
This means that community pharmacy teams will need to provide AAH with the details of a patient’s prescription before an order can be placed.
AAH will then review the request before approving the order, the supply notification said.
For Varilrix, general practices will be limited in the amount they can order, and will only need to provide prescription details if they override the quota.
Clinicians in any primary care setting must go through a prescription validation system when ordering Varivax from Alliance Healthcare.
This is the latest in a string of shortages, which the Pharmaceutical Services Negotiating Committee said in July 2022 has become “a critical situation” for community pharmacy teams.
In August 2022, more than half of pharmacists responding to The Pharmaceutical Journal’s annual salary and job satisfaction survey said that medicine shortages have put patients at risk “in the past six months”.
“In the UK varicella vaccination is not routinely offered as part of the UK childhood schedule but is recommended for specific groups as a selective pre-exposure vaccination strategy,” the supply notification said, including “immunocompromised individuals, neonates and pregnant women”.
“The current UK selective programme aims to protect those at highest risk of severe disease from exposure,” the notification continued but added that, owing to supply shortages, “the UKHSA [UK Health Security Agency] recommends prioritising available stock for those at greatest risk from severe disease”.
It specified that these include susceptible adult and child household contacts of immunocompromised individuals and non-immune healthcare workers working with severely immunosuppressed patients, with susceptibility confirmed by quantitative antibody testing.
“During the current shortage, vaccination of other healthcare workers and laboratory workers should be deferred,” the notification added.