Anna Watson / Alamy Stock Photo
Patients attending private menopause clinics are being prescribed oestrogen at up to double the recommended dose, The Pharmaceutical Journal has learnt.
Experts say they are worried that, in some cases, women not being prescribed a sufficient dose of progesterone alongside oestrogen to adequately protect the womb lining, which can lead to an increased risk of some types of cancer.
The concerns over “unorthodox” prescribing at private clinics comes amid rising awareness of the symptoms of the menopause, with the number of hormone replacement therapy (HRT) products prescribed in England increasing by a third in one year, and shortages of products ongoing since 2018.
The British Menopause Society (BMS), the society for healthcare professionals and others specialising in post reproductive health, told The Pharmaceutical Journal that it was aware of issues regarding the prescribing of private providers and was addressing the matter “as a priority”.
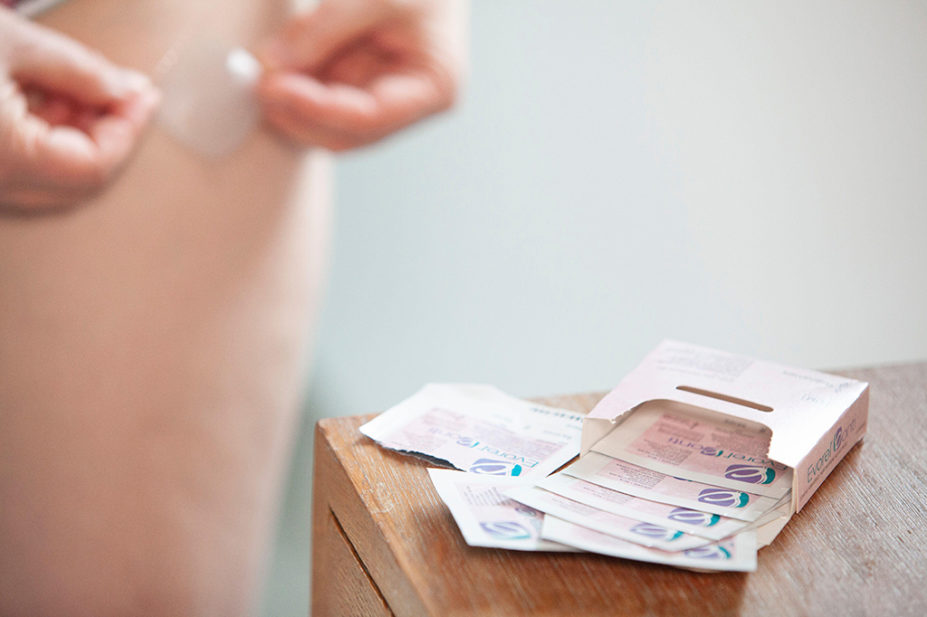
However, those running private clinics specialising in menopause explained that some women need higher doses of oestradiol because absorption of oestrogen through the skin is “variable”.
The Pharmaceutical Journal has spoken to several pharmacists and nurses, who say they have encountered patients on very high doses of oestrogen as HRT.
Brendon Jiang, a senior clinical pharmacist for North Oxfordshire Rural Alliance Primary Care Network (PCN), said that his team were increasingly getting letters from private clinics requesting for patients to be prescribed doses of oestrogen that exceed licensed recommendations.
“We have seen more requests for doses that are off-label and outside national and international guidelines. We advise prescribers against assuming clinical responsibility and have refused, writing back to private providers insisting that they continue prescribing.”
“The worst I’ve seen is double — so 200 micrograms — the max 100 microgram Evorel patches,” he added. “And that was in addition to vaginal oestrogen and topical gel being used too.”
Jiang said he had also heard that, in some of cases of overprescribing, the patient was not being prescribed an adequately protective dose of progesterone.
“I have seen recommendations to increase the oestrogen dose while simultaneously reducing progesterone below recommended minimum levels,” he explained, adding that there were instances where patients, who were refused a prescription by their GP, had then successfully got a prescription from a private clinic.
“We have seen women with contraindications to HRT, seeking and obtaining supplies from private clinics,” he added.
Nuttan Tanna, pharmacist consultant, women’s health and osteoporosis, at London North West University Healthcare NHS Trust, said she had received similar feedback in her region: “[We have] referrals for bleeding investigations and then find the patient was on very large doses [of oestrogen] prescribed previously by private providers.”
Debra Holloway, a gynaecology nurse consultant and co-lead of a specialist menopause service at Guy’s and St Thomas’ NHS Foundation Trust, echoed these concerns: “We do see some women in clinic who are on large amounts of oestrogen and often smaller or inadequate amounts of progestogens.
“I do get advice and guidance queries from GPs about the amounts of oestrogen prescribed, so it is not uncommon.”
In a joint statement to The Pharmaceutical Journal, Paula Briggs, current chair of the BMS, and Haitham Hamoda, immediate past chair, said that the society was “very much aware” of the issues surrounding “unorthodox prescribing” by private providers.
“We have been contacted by significant numbers of members, non-members and women questioning the prescribing that falls out with national guidance and licensing recommendations.
“We are working with other specialist societies and colleges — who share our concerns — to address this matter as a priority.”
“It’s unusual that you would need higher doses [of oestrogen than licensed recommendations],” Hamoda told The Pharmaceutical Journal. “If you’re [prescribing] that, you need to question: why is there a need for it?
“In the exceptional cases where you are [prescribing] a higher dose, you need to make sure that the progesterone is opposing what you’re giving. If you’re putting someone on higher doses of oestrogen and you’re not opposing that properly, you are putting the patient at an increased risk of hyperplasia and cancer.”
Responding to the concerns, Magnus Harrison, chief medical officer at Newson Health Group — the largest private menopause clinic in the UK — explained that some women need higher doses of oestradiol because absorption of oestrogen through the skin is “variable”.
“Some women need higher than the licensed doses to achieve a physiological level of oestradiol,” he added. “Blood levels of oestradiol are monitored for patients at Newson Health clinics.”
Harrison said that, for younger women with premature ovarian insufficiency, studies have shown that higher doses of oestradiol are needed to achieve a physiological response.
“It is important to provide adequate oestradiol to perimenopausal and menopausal women to improve symptoms, and more importantly to improve future health. Low oestradiol levels in the menopause are associated with increased risk of diseases such as cardiovascular disease, osteoporosis, type 2 diabetes and dementia.
He added that the data regarding actual dose of progesterone to provide adequate endometrial protection is “not robust”.
“At Newson Health clinics, we ensure that women are regularly followed up, at least annually, and we ask that any vaginal bleeding is reported.
“We follow ‘The British Menopause Society & Women’s Health Concern 2020 recommendations on hormone replacement therapy in menopausal women’ — the HRT dosage regimen and duration should be individualised with annual evaluation of advantages and disadvantages.”


