Shutterstock.com
A new “wafer” form of naloxone is under development by researchers at King’s College London after receiving part of a £12m package of funding from the government.
In a statement published on 18 October 2024, the government said the funding is targeted at developing new technology to support people with drug addictions.
The statement said part of this funding will “support research to improve the accessibility of the life-saving drug naloxone”.
“King’s College London is developing rapid-dispersal naloxone wafers to improve the accessibility and portability of this life-saving emergency antidote medication,” the statement said.
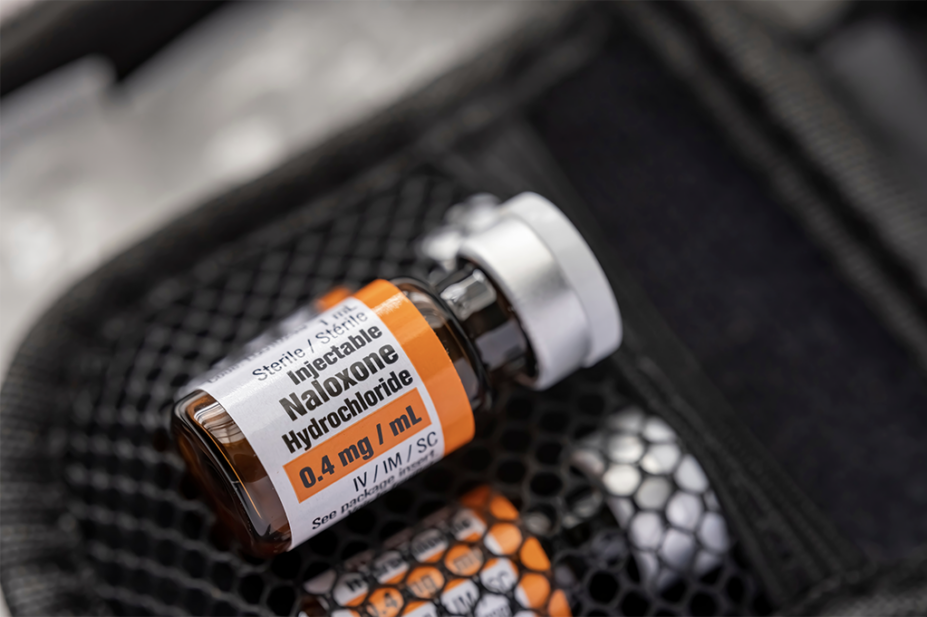
“Naloxone rapidly reverses heroin or opioid overdose, but current forms (injection and nasal spray) have limitations, such as requiring healthy nasal passages and consistent carrying by users.”
It added that the wafers being developed will “disintegrate within seconds” and can easily fit into a wallet, addressing “the current low carriage rates which are around 15–20%”.
The research is one of 11 projects selected across the UK as part of the Reducing Drug Deaths Challenge and the £10m National Institute for Health and Care Research’s (NIHR) Addiction Mission: Innovation for Treatment and Recovery Awards, which are being run in partnership with the Scottish government and the NIHR.
The government announced in May 2024 that it will “shortly update legislation” to allow pharmacists and pharmacy technicians to provide take-home supplies of naloxone without a prescription.
The amendments to legislation were approved in the House of Commons and House of Lords on 23 October 2024.
Commenting on the naloxone project, Amira Guirguis, chair of the Royal Pharmaceutical Society’s science and research committee, said the wafers offer “an innovative approach to tackling opioid overdose, particularly by making this life-saving drug more accessible in emergency situations”.
“A discreet, portable wafer that dissolves quickly in the mouth could provide a practical alternative to existing injectable and intranasal formulations, which require either training or optimal nasal conditions for effective use,” she said.
However, she added that “a key area for this research will be to assess the bioavailability of the wafers compared to existing naloxone formulations”.
Guirguis also noted some possible practical challenges with using wafers, including that “in opioid overdoses, patients often experience dry mouth due to dehydration, which could delay the wafer’s dissolution”.
“In contrast, nasal sprays and injections can be administered without direct contact, which may make them more straightforward in emergency settings,” she said.
Graham Parsons, director of pharmacy at Waythrough, a charity supporting people with mental health, drugs, alcohol, housing and related areas, welcomed the research.
“These innovative approaches to reducing drug-related harm are a step in the right direction to saving more lives,” he said.
However, Catriona Matheson, professor in substance use at the University of Stirling and former chair of the Scottish government’s Drug Deaths Taskforce, said she was “ambivalent” about the development of the wafers.
“I am not wholly convinced it is needed as we have intranasal and intramuscular [formulations], both of which are effective. I suppose a range of options is good if it proves to show reasonable bioavailability,” she said.
In October 2023, a naloxone emergency supply service was added to the Scottish community pharmacy public health service, meaning every community pharmacy in the country is required to stock an emergency naloxone supply.
In the same year, there were 1,172 deaths related to drug misuse in the country, of which 80% were related to opioids.
1 comment
You must be logged in to post a comment.

I believe more important than newer formulations of naloxone is access at community level. The concept of emergency supply of naloxone by community pharmacies in Scotland as a public health funded intervention would be a rational approach if agreed across England and Wales, improving access!