Shutterstock.com
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus
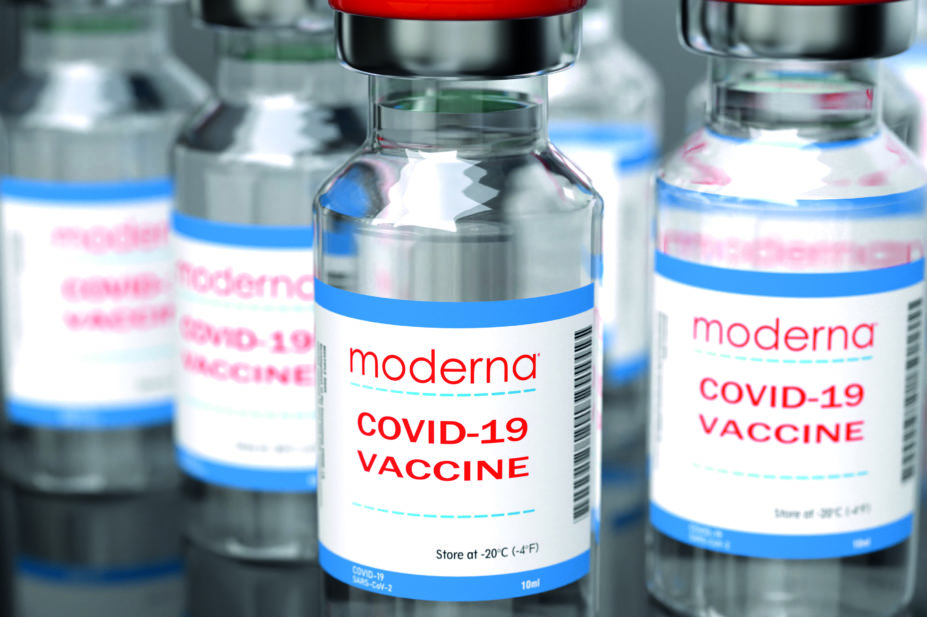
A third COVID-19 vaccine has been authorised for temporary supply in the UK by the Medicines and Healthcare products Regulatory Agency (MHRA) after meeting the required standards of safety, efficacy and quality.
The mRNA vaccine developed by Moderna, which has already been granted a conditional marketing authorisation in the EU, was found to be 94% effective in preventing COVID-19, including in the older population.
The government has agreed to purchase an additional 10 million doses of the Moderna vaccine on top of its previous order of 7 million, taking the total number of doses to 17 million.
Doses of the vaccine will be available in the UK from spring 2021 and the Joint Committee on Vaccination and Immunisation will submit updated advice on which groups to prioritise for vaccination before doses become available.
The vaccine, which is indicated for individuals aged 18 years and over, is administered in a two-dose regimen, with the second dose administered 28 days after the first.
The Department of Health and Social Care said the Moderna vaccine will be deployed in a similar method to the Pfizer/BioNTech and Oxford/AstraZeneca vaccines: through hospital hubs for NHS and care staff, and older patients; local community services; and vaccination centres across the country.
“This is further great news and another weapon in our arsenal to tame this awful disease,” said Matt Hancock, health and social care secretary.
“We have already vaccinated nearly 1.5 million people across the UK and Moderna’s vaccine will allow us to accelerate our vaccination programme even further once doses become available from the spring.
“While we immunise those most at risk from COVID-19, I urge everyone to continue following the rules to keep cases low to protect our loved ones.”
The safety and efficacy of Moderna’s COVID-19 vaccine was evaluated in an ongoing phase 3 randomised controlled trial conducted in the United States involving 30,351 participants, aged 18 years and older, who received at least one dose of vaccine (n=15,185) or placebo (n=15,166) and were followed for a median of 92 days.
The trial showed a 94.1% reduction in the number of symptomatic COVID-19 cases in the people who received the vaccine, compared with people who received placebo, meaning that the vaccine demonstrated a 94.1% efficacy in the trial.
The trial also showed 90.9% efficacy in participants at risk of severe COVID-19, including those with chronic lung disease, heart disease, obesity, liver disease, diabetes or HIV infection. The high efficacy was also maintained across genders, racial and ethnic groups.
The approval of the Moderna vaccine follows the approval of two other COVID-19 vaccines manufactured by Pfizer/BioNTech and Oxford/AstraZeneca in December 2020.