Nephron / Wikimedia Commons
Researchers have developed a hydrogel that selectively delivers drugs to inflamed tissue, which could pave the way for improved treatment options for patients with inflammatory bowel disease (IBD), according to a study published in Science Translational Medicine
[1]
on 12 August 2015.
A team of Massachusetts-based researchers produced the inflammation-targeting hydrogel (IT-hydrogel), made from ascorbyl palmitate, whose microfibres encapsulated the corticosteroid drug dexamethasone, then tested it in a mouse model.
The hydrogel was delivered by enema and, as it carried a surface negative charge, adhered to the inflamed mucosa of colitic mice, which are covered in positively charged proteins.
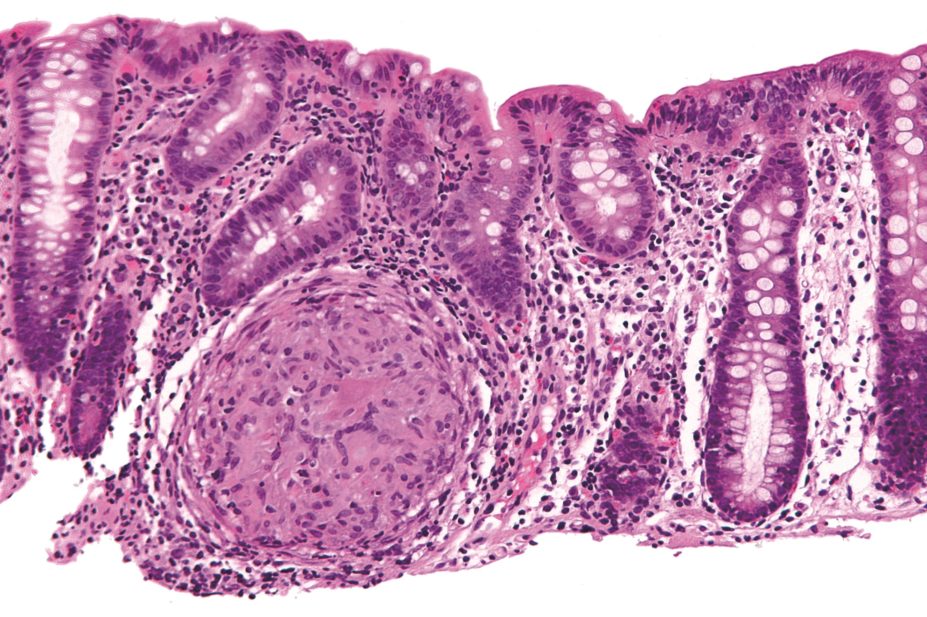
Enzymes derived from inflammatory cells degraded the gel, which released the drug directly on to the site of inflammation. Compared with delivery of the free drug by enema, IT-hydrogel was more effective at reducing inflammation in murine intestinal tissue.
“Our study provides proof of concept for IT-hydrogel as a safe and potentially effective drug delivery platform for colonic IBD and other inflammatory diseases,” say the researchers.
Around 240,000 people in the UK have IBD. Ulcerative colitis and Crohn’s disease are the two most common forms.
Almost all patients with ulcerative colitis will require enema-based therapy at some point in their treatment. However, enema therapy has three major issues: it is difficult to retain; there is high systemic absorption of the drug that can lead to toxic side effects; and compliance is low as patients must take enemas every day.
“Our approach can potentially address all three. The engineered gel that we designed has dual targeting capability. It rapidly attaches to ulcers within seconds to minutes − we have five to ten times less systemic absorption as the gel only attaches to ulcers − and selectively releases drug in the presence of ulcers, and we showed that we could reduce the dosing frequency,” says corresponding author Jeff Karp, associate professor of medicine, Brigham and Women’s Hospital, Harvard Medical School.
However, the researchers say further pre-clinical development will be needed. Report co-author Giovanni Traverso − gastroenterologist and biomedical engineer at Massachusetts General Hospital, Harvard Medical School – says: “We’re optimistic that the inflammatory-targeting hydrogel, which is made of constituents found in food, will likely be safe. Furthermore, further validation in other disease colitis models will have to be proven to ensure the observations are consistent. These may include different rodent models of colitis or other larger animal models of IBD.”
The British Society of Gastroenterology says IBD is a debilitating disease with a massive impact on social care. “Enemas are difficult to tolerate, so if the application of hydrogel technology described in this study can deliver a more targeted approach to this form of treatment then it is encouraging news. We look forward to further clinical trials, but it is an exciting development,” says Ayesha Akbar, consultant gastroenterologist at St Mark’s Hospital, London, speaking on behalf of the organisation.
Drugs to suppress the immune system are the mainstay of medical management of IBD, though nutritional therapy as primary treatment is also important in Crohn’s disease. However, 30% of patients fail to respond to these drugs and will then be considered for anti-TNFα biological therapies or surgery[2]
. The cost of IBD to the NHS is estimated to be around £720m annually.
References
[1] Zhang S, Ermann J, Succi MD et al. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Science Translational Medicine 2015;7:300ra128.
[2] The IBD Standards Group. Quality Care: Service Standards for the healthcare of people who have Inflammatory Bowel Disease (IBD), 2009.