Alisdair Macdonald
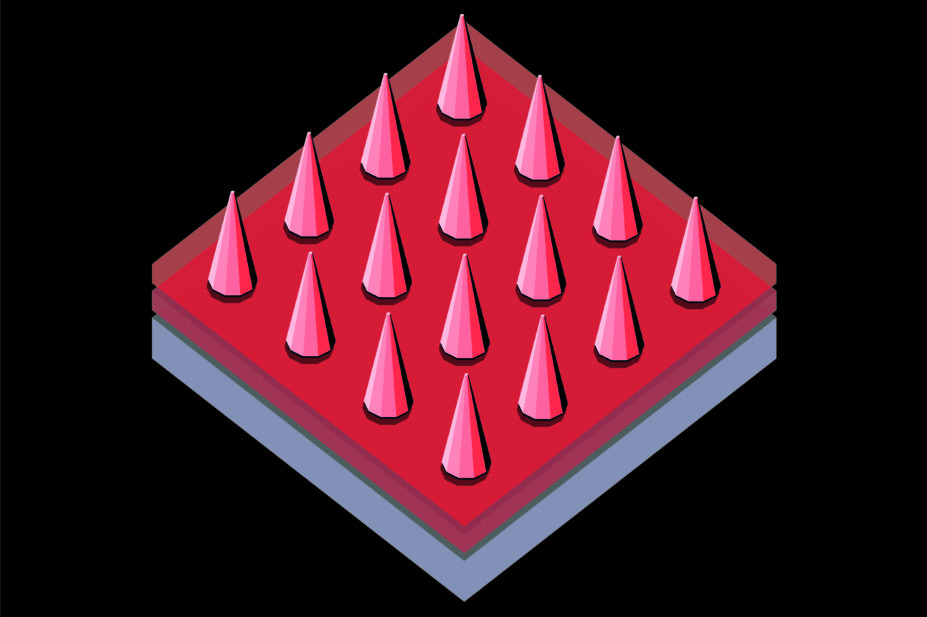
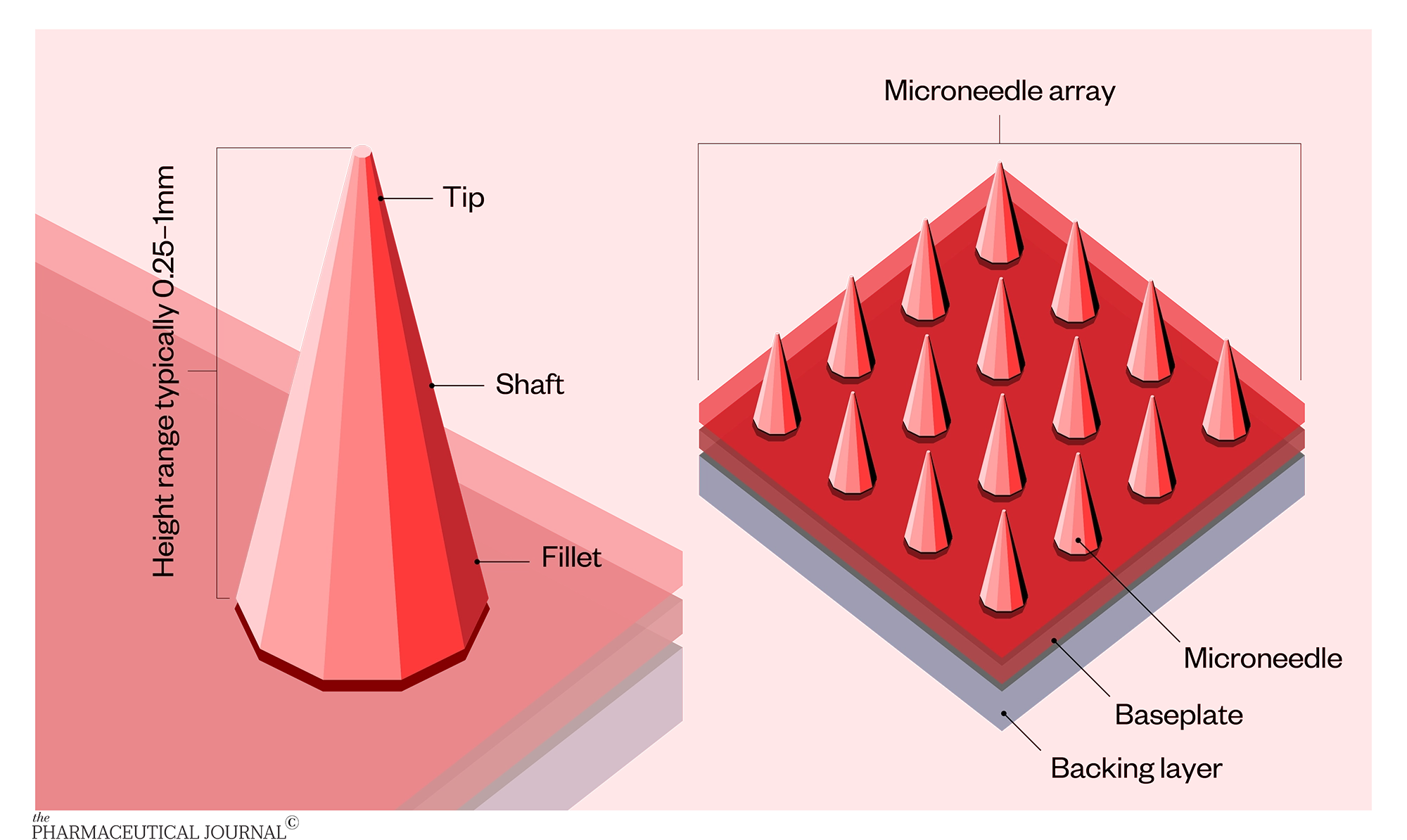
Described as a hybrid between a hypodermic needle and a transdermal patch, microneedles are tiny needles (less than 1mm) made of polymer, metal, silicon, glass or ceramic. Anywhere from one to thousands of needles are fixed to a baseplate or backing layer. The vaccine or drug is generally placed in or on the microneedle tip.
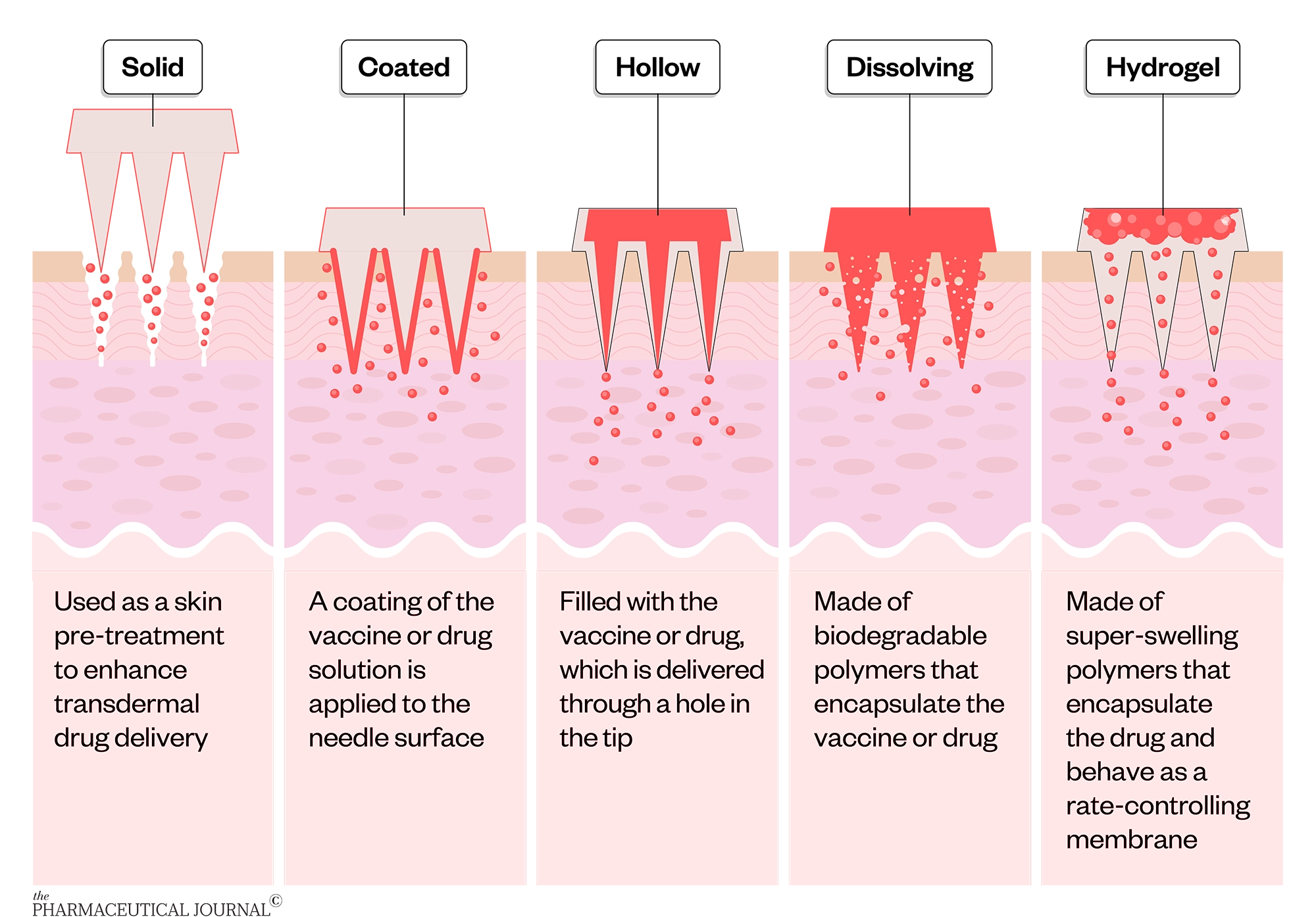
Five main types
Microneedles are typically classified into five types:
- Solid
- Coated
- Hollow
- Dissolving
- Hydrogel
Challenges with solid and coated microneedles have inspired the development of other types of microneedles. Hollow microneedles can deliver liquids, rather than requiring dry formulations, and allow relatively high doses to be administered. Dissolving microneedles benefit from simple and low-cost fabrication (although mass production has proven challenging), precise and consistent dosing, relatively higher vaccine dose capacity, controlled drug delivery and ease of disposal. Hydrogel microneedles are the newest type of microneedles and deliver relatively higher doses compared with other dry microneedle dosage forms, and theoretically leave no polymer residue within the skin.
Advantages over other delivery systems
Microneedles are painless, less invasive than traditional injections, easy to self-administer and do not require cold storage.
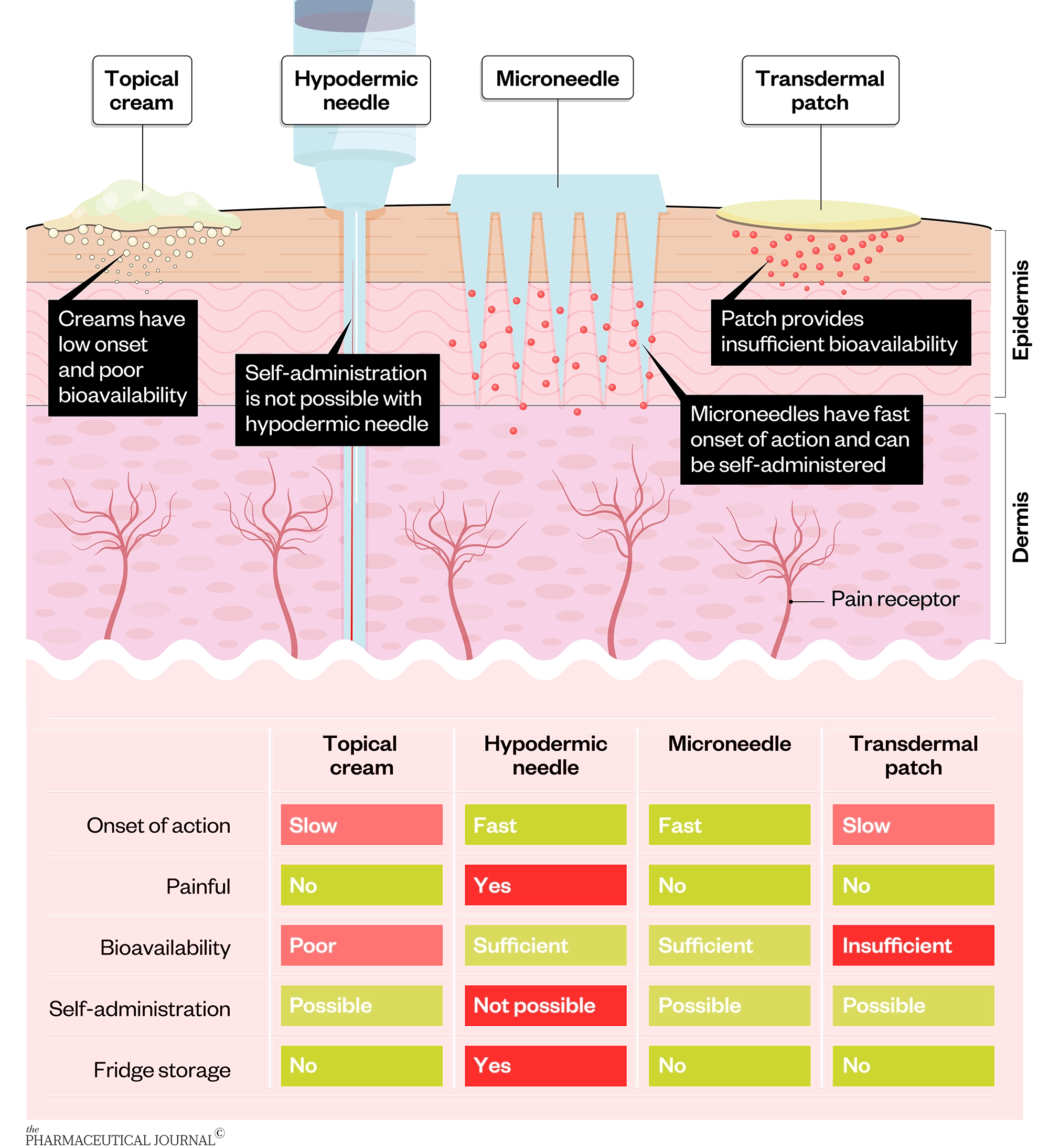
Other advantages include:
- Long-lasting immunity: An extended-delivery profile can be achieved by administering multiple vaccine doses via microneedles, resulting in a long-lasting immune response compared with injections;
- Cold chain: A huge advantage for vaccine delivery via microneedles is that the cold chain can be eliminated because they do not need to be stored in the fridge or freezer, something that has been extremely challenging during the roll-out of currently approved COVID-19 vaccines;
- Dose sparing: Significant dose sparing has been demonstrated with the delivery of various vaccines via microneedles, with some microneedles using only 4% of a subcutaneous dose to stimulate a similar immune response.
Why vaccine delivery?
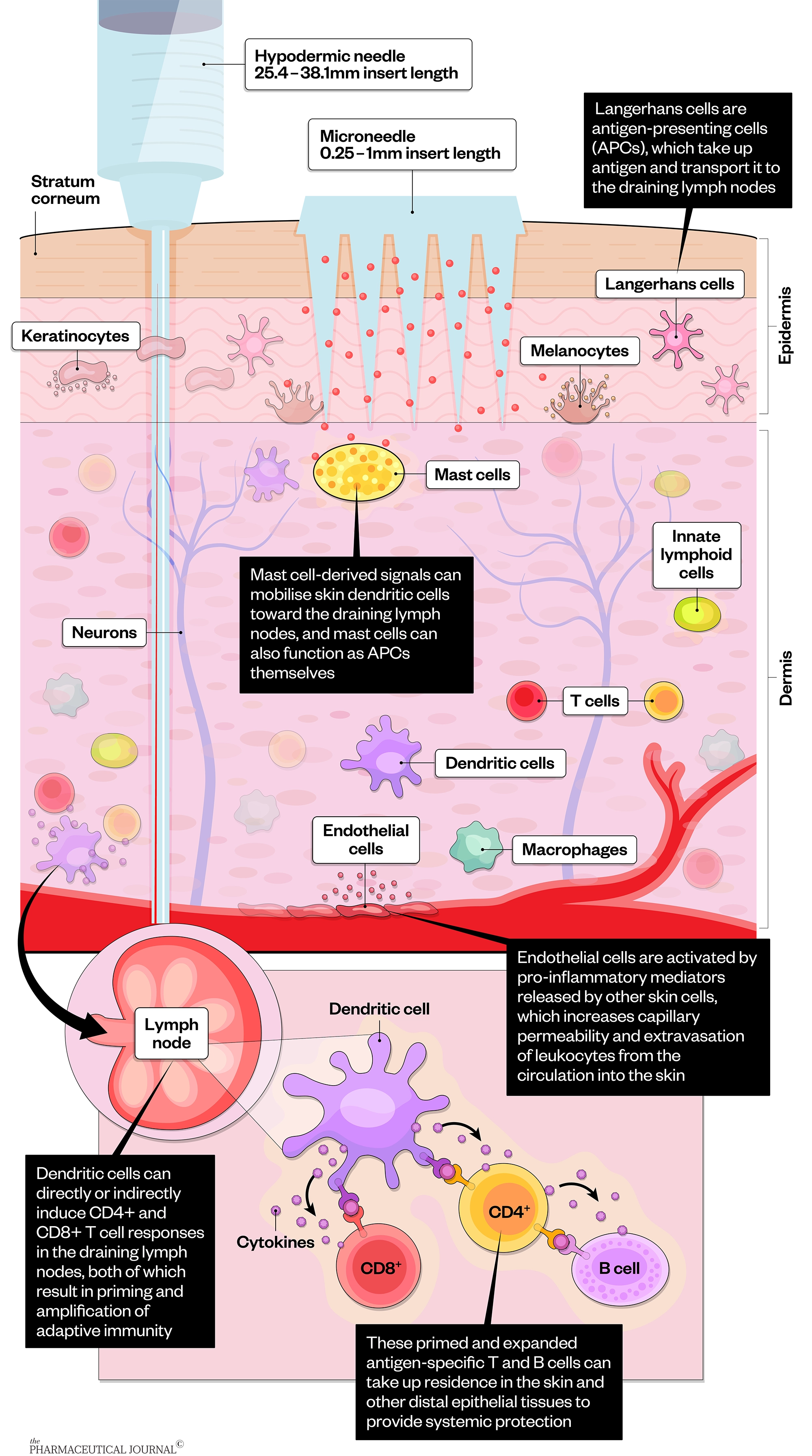
Microneedles generate temporary micron-sized pores through which vaccines and drugs that cannot normally be administered via the skin can be delivered. Microneedles penetrate the skin barrier — the stratum corneum — and deliver their load directly to the dermis, which has a rich supply of blood and lymph vessels, meaning it is exposed to various types of immune cells that play a critical role in the induction of immune responses. Compared with subcutaneous and muscle tissues, the more immune-responsive microenvironment present in the skin makes microneedles particularly effective at delivering vaccines.
Delivering COVID-19 vaccines
Microneedles have been used to successfully administer a variety of vaccine types, including viral vector, DNA, protein, peptide, virus-like particles, nanoparticle vaccines and attenuated or inactivated viruses, with or without adjuvants.
The most extensively studied is the influenza vaccine, but vaccines for hepatitis B, hepatitis C, measles, rubella, Japanese encephalitis, HIV, herpes simplex virus, human papillomavirus, rabies, Zika, rotavirus, polio, chikungunya, respiratory syncytial virus, West Nile fever, dengue, Ebola, Middle East respiratory syndrome and COVID-19 have also been tested in animals, and generally induce virus-specific humoral and cellular immune responses, either superior or comparable to those elicited by hypodermic needle-based vaccination.
Clinical trials of vaccines for influenza, rabies, polio, hepatitis B and varicella-zoster virus have also shown promising results.
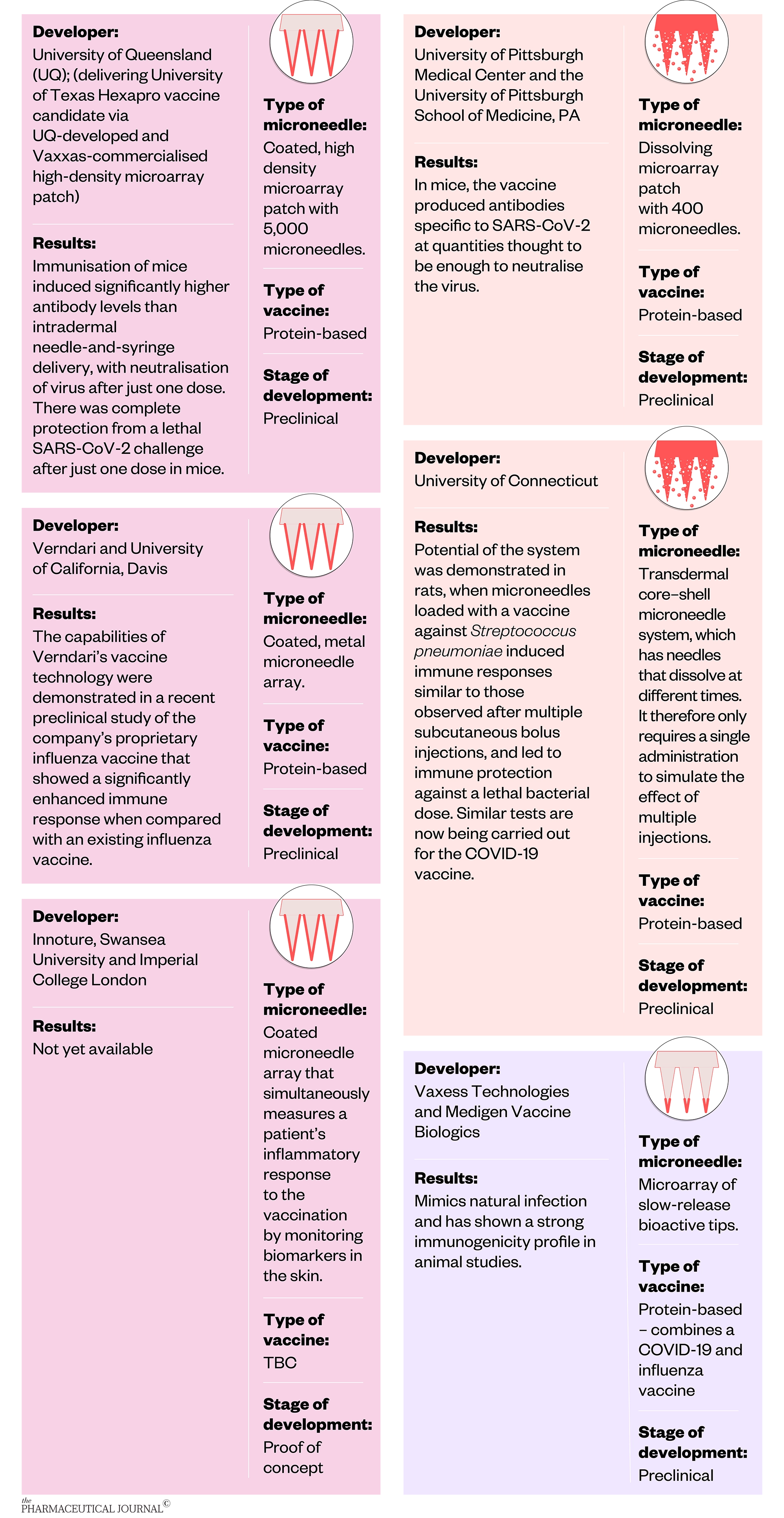
The following research groups are currently working on developing microneedles for delivering vaccines against COVID-19:
Editorial advisers: Fiona Smith, PhD researcher, CDT for Transformative Pharmaceutical Technologies, School of Pharmacy, University of Nottingham; Lalitkumar Vora, postdoctoral research fellow, School of Pharmacy, Queen’s University Belfast
Infographics: Alisdair Macdonald