Shutterstock
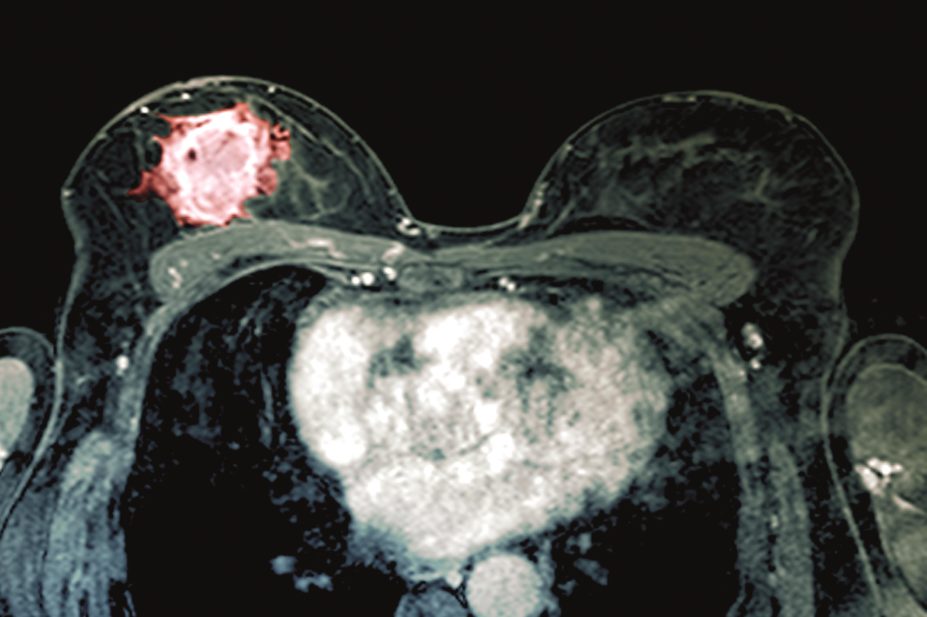
Women with type 2 diabetes who take glucagon-like peptide-1 (GLP-1) analogues do not have a higher risk of breast cancer than those who take dipeptidyl peptidase-4 (DPP-4) inhibitors, results from a cohort study suggest.
Using data from the UK Clinical Practice Research Datalink involving 44,984 women who were newly treated with glucose-lowering drugs between 1 January 2007 and 31 March 2015, researchers found that breast cancer rates did not differ between those taking GLP-1 analogues and DPP-4 inhibitors over a mean follow-up of 3.5 years.
The researchers say their findings should help allay concerns raised by regulatory agencies in 2014[1]
about a potential link between the drugs and breast cancer following the results of early trials of the GLP-1 analogue liraglutide, which reported greater incidences of breast cancer in treatment versus placebo arms.
Reporting their findings in The BMJ
[2]
(online, 20 October 2016), the team found that there was a similar level of breast cancer risk among the 498 patients who used GLP-1 analogues, such as liraglutide and exenatide, and the 2,422 patients who used DPP-4 inhibitors, at 4.4 and 3.4 per 1,000 person-years, respectively.
When the researchers factored in duration of use in secondary analyses, they did detect an increased risk of breast cancer when patients had used GLP-1 analogues for between 2.1 and 3.0 years (hazard ratio [HR] 2.7) and when the time since initiation was between 3.1 and 4.0 years (HR 2.6). But both of these associations disappeared at later time points.
“It seems these atypical duration patterns are most likely a result of detection bias,” says lead author Blánaid Hicks from McGill University, Montréal, Canada. “This occurs in observational studies such as this, when one exposure group might have a greater or lower opportunity of being screened for the outcome than another exposure group.”
In support of this, the researchers showed that the risk of breast cancer was only elevated among GLP-1 users who had no history of mammography screening but not in those who had previously been screened. They say this is compatible with a potential enhanced detection of prevalent breast cancer.
“The mechanism for this detection is not well understood,” says Hicks. “It has been proposed that weight loss associated with GLP-1 agonist use may lead to better detection of breast lumps but evidence to support this association of weight loss and detection is lacking.”
The researchers also note that findings from the largest randomised trial of liraglutide — the LEADER trial[3]
, published in June 2016 — did not indicate an elevated risk of breast cancer associated with the drug.
But Hicks says that although the results of her team’s research should be reassuring to patients, further observational studies are needed with a longer follow-up period. She also says prescribers should continue to discuss the potential risk of breast cancer with their patients until there is more evidence.
“Although [detection bias] is a likely explanation, we cannot rule out that these are true increases in risk caused by the promotion of breast tumour growth by GLP-1 agonists,” she says.
Elizabeth Robertson, director of research at the charity Diabetes UK, agrees that although the authors’ findings probably result from better detection, a potential biological explanation cannot be dismissed.
“We would welcome further research which investigates this observation and the underlying mechanisms. It is important that people who are prescribed GLP-1 analogues continue to take them and contact their diabetes team if they have any concerns.”
References
[1] US Food and Drug Adminstration. Analysis of cancer incidence observed in clinical trials of liraglutide. 2016. Available at: www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm416154.pdf
[2] Hicks BM, Yin H, Yu OHY, et al. Glucagon-like peptide-1 analogues and risk of breast cancer in women with type 2 diabetes: population based cohort study using the UK Clinical Practice Research Datalink. BMJ 2016;355:i5340. doi: 10.1136/bmj.i5340.
[3] Marso SP, Daniels GH, Brown-Frandsen K, et al. LEADER Steering Committee LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311-22. doi:10.1056/NEJMoa1603827.