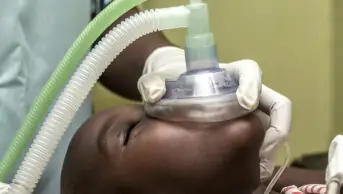
BURGER / PHANIE / SCIENCE PHOTO LIBRARY
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus
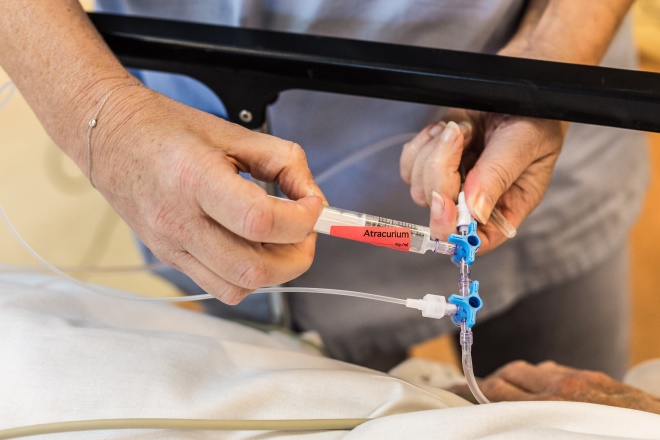
Source: BURGER / PHANIE / SCIENCE PHOTO LIBRARY
The neuromuscular blocking agents atracurium and cisatracurium are used first line in the management of COVID-19 patients
Supplies of certain drugs used when intubating patients with COVID-19 will run out “over the coming days”, the government has warned.
In a supply disruption alert issued on 16 April 2020, the Department of Health and Social Care (DHSC) said there were “limited supplies” of all strengths of the neuromuscular blocking agents atracurium and cisatracurium, owing to a “recent increase in demand”, with trusts asked to switch to using rocuronium or pancuronium when supplies deplete.
Atracurium and cisatracurium are listed by the Royal College of Anaesthetists as “first line” drugs in the management of COVID-19 patients. They are potent muscle relaxants that are typically used during surgery and in patients that are ventilated in critical care.
While supplies of rocuronium “remain available at present”, the alert asks trusts to consider using suxamethonium when performing a tracheal intubation as part of a rapid sequence induction “to preserve supplies of rocuronium”.
To mitigate supply issues in the UK, the DHSC said in the alert that it will be distributing the “remaining stock of atracurium and rocuronium injections” to trusts based on “recent modelling data which takes account of current stockholding and intensive care unit (ICU) bed occupancy”.
“Stocks of cisatracurium in the supply chain are critically low and restock is not expected in the near future,” it said, adding that remaining supplies are not sufficient to be centrally distributed.
The DHSC advised pharmacy procurement and clinical teams at NHS trusts to only purchase up to the amount of stock that their regional procurement lead has allocated to them, and work with their medicines safety officer to consider how stock will be managed safely within the trust.
Accord Healthcare, which manufactures cisatracurium, said in a statement published on 17 April 2020 that the surge in demand for its product “was anticipated … when the COVID-19 pandemic first emerged”.
Tony Cordrey, vice-president of operations at Accord Healthcare, said: “In response to growing UK and global demand for vital COVID-19 drugs, including ICU medicines, such as cisatracurium, midazolam and paracetamol, we are working closely with regulatory agencies to accelerate approval for a large new manufacturing facility that can further increase output of these medicines.”
He added that the new facility “will be one of the largest pharmaceutical manufacturing sites in Asia”.
A spokesperson for the company told The Pharmaceutical Journal that the manufacturer has “increased batch sizes by 250%” by dedicating its production facility to intensive care medicines, producing 30mL vials rather than a range of 1mL, 2mL or 5mL vials.
This comes after the European Medicines Agency said, on 6 April 2020, that it was considering regulatory actions to support increased manufacturing capacities in response to the pandemic, including “speeding up the approval of a new manufacturing line or site”.
Ravi Mahajan, president of the Royal College of Anaesthetists, told The Pharmaceutical Journal that the College was aware of possible increased demand on the supply of these drugs, adding that their use would have to be carefully managed to ensure that those patients that need them most, have access to them.
“The College had already put out guidance aimed at minimising the use of these drugs in anaesthesia while always maintaining the safety of patient care and making suggestions about the use of alternative drugs that are less likely to encounter supply problems,” he said, adding that it plans to publish further guidance on this issue.
The guidance, which it says was drawn up along with The Faculty of Intensive Care and “working closely with the chief pharmaceutical officer at NHS England” was published on 2 April 2020 and lists 12 “first line” medicines alongside alternative medicines that can be used to manage COVID-19 patients.
A British Medical Association survey, conducted between 14 and 16 April 2020, revealed that 40% of 4,273 doctors have been forced to provide treatments that they felt to be less effective because the treatment they would normally use was in short supply.
A spokesperson for the DHSC told The Pharmaceutical Journal: “We know how distressing shortages can be, but we want to assure people that we have well-established processes to manage and mitigate supply problems.
“Alternative medicines are available, and we are working closely with clinical bodies to issue guidance to hospitals and source further supplies for the UK.”