Shutterstock.com
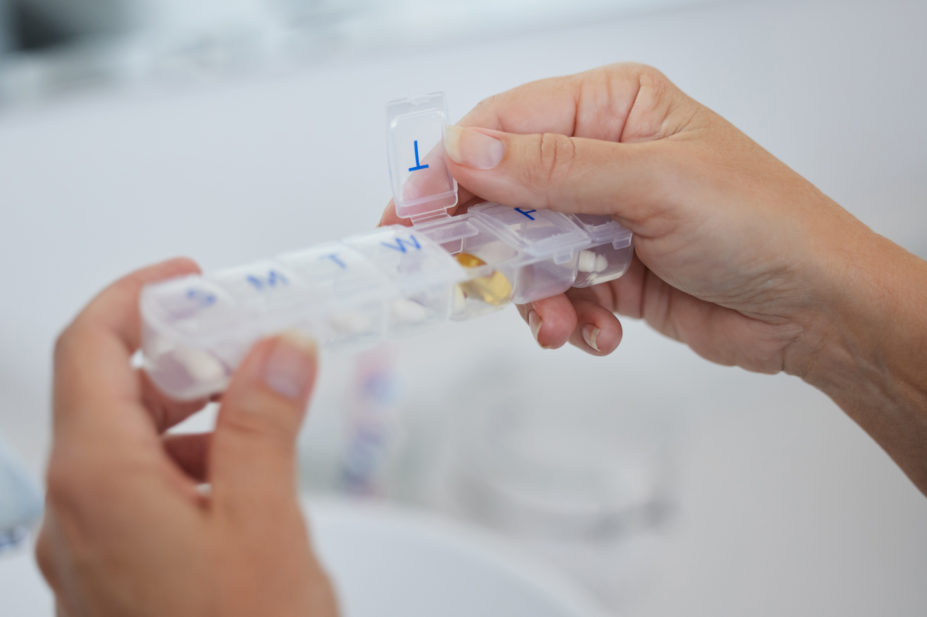
The majority of NHS acute hospital trusts providing multi-compartment compliance aids (MCAs) in England have “frequent difficulties” with them, according to the results of a national survey published in the British Journal of Clinical Pharmacology.
According to the authors, this lack of a consistent approach to MCA use in England could be increasing the risk of medicines-related harm in the vulnerable populations that rely on them.
The questionnaire was distributed by the NHS Specialist Pharmacy Service to chief pharmacists at every acute trust in England, with questions covering policy, initiation, supply and review of MCAs, alternatives offered, and pharmacy staffing and capacity related to MCAs.
Responses were received from 72 (52%) of the acute trusts, with the majority of responses completed by clinical pharmacists (43%), followed by chief pharmacists and pharmacy technicians (18% and 15%, respectively).
In total, 55 of the trusts who responded (76%) dispensed MCAs.
The responses showed that, at least once a week, 85% of the 55 trusts that dispensed MCAs (47) found that not all medication could be dispensed into an MCA.
The time it takes to prepare an MCA was also considered a significant issue, with 62% (34) of trusts reporting that it delayed discharges.
In addition, 60% (33) reported that changes were often made to the discharge prescription after the MCA had been prepared and 47% (26) felt that, at least weekly, discharge prescriptions could not be completed on the day of receiving the order.
The most common reason for initiation of an MCA was at the request of a care agency, with free-text responses showing that some care agencies would only accept the patient if their medicines were supplied within an MCA.
The least common reason for MCA initiation was patient request, which highlighted a “worrying trend” that patient choice and informed consent were often not prioritised prior to starting an MCA, the authors said.
The results also showed that a significantly shorter supply of medicines was often given on discharge for patients with MCAs, compared to those without — 73% of trusts dispensed 7-day prescriptions compared to a 14- or 28-day prescription.
The authors said that the overriding pharmacy perception was that there were infrequent reviews of the suitability of patients’ MCAs during inpatient and outpatient visits and that all healthcare professionals were “infrequently reviewing” the appropriateness of MCAs.
Overall, they concluded that a national evidence-based approach to the use and provision of MCAs, with increased emphasis on patient choice, was required.
“The impact on the NHS is far-reaching, with potential increased costs associated with workload, the management of MRH [medicines-related harm], and delayed discharges,” they said.
Andre Yeung, local professional network chair (pharmacy) at NHS England and NHS Improvement, North East and Yorkshire, welcomed the findings of the study.
“MCAs are provided by a myriad of providers of pharmacy services including community pharmacies and hospital pharmacies,” he said.
“In my view, the varying approach to the use of MCAs is due to a lack of standards and quality assurance and investment in this area. This means that the rules of the game aren’t clear and therefore providers do what they think is right for each patient but the results across the system are hugely variable and lead to poorer quality outcomes.”
Yeung said he thought there was a “huge need” for national guidance and an associated mechanism to ensure the quality provision of medicines compliance support by pharmacies and pharmacists.
“This will undoubtedly mean hospitals and community pharmacies, as well as regulators and commissioners, working together to agree set standards around the provision of MCAs.
“One would imagine that this will involve stipulating that an MCA should not be used unless there is a clear reason to do so. And in turn, this will mean that the evidence of the effectiveness of MCAs will need to be ‘proven’ and a standardised method of assessing patients developed that has demonstrable benefits to patients.”
Read more: Think outside the box: replacing multicompartment compliance aids
You may also be interested in

Medicines commission calls for greater clarity on risk of suicidal behaviour from antidepressants

Patient safety commissioner pushes government on valproate redress almost two years after report
