Christine Whitehead / Alamy Stock Photo
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/coronavirus
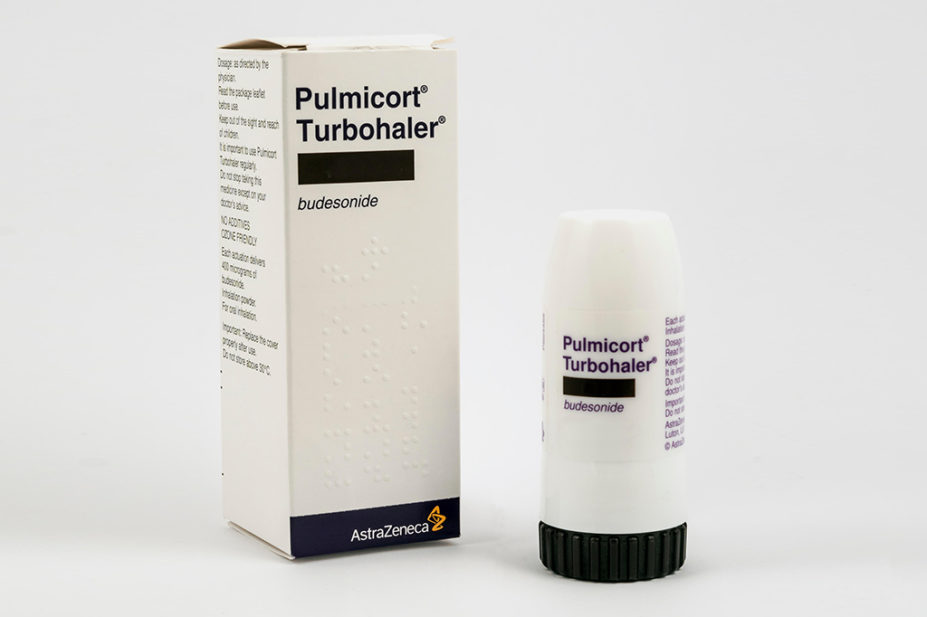
Inhaled budesonide should be considered for patients with COVID-19 who are at higher risk of complications in the community, according to results from a UK-wide clinical study.
The ‘Platform Randomised Trial of Treatments in the Community for Epidemic and Pandemic Illnesses’ (PRINCIPLE) is the first randomised trial to demonstrate effectiveness of inhaled budesonide to treat COVID-19 in the community.
It builds on earlier evidence from the phase II ‘Steroids in COVID-19’ (STOIC) trial, which found that early administration of inhaled budesonide reduced the likelihood of needing urgent medical care and reduced time to recovery after early COVID-19.
Findings from the PRINCIPLE trial, published in the Lancet on 10 August 2021, suggested that inhaled budesonide improves recovery time and could potentially reduce hospital admissions or death.
Eligible participants included those who were aged 65 years or older, or aged 50 years or older with comorbidities, who had been unwell for up to 14 days with suspected COVID-19, but had not been admitted to hospital.
Overall, 4,700 participants were randomly assigned to either usual care (n=1,988), usual care plus 800μg of inhaled budesonide twice daily for 14 days (n=1,073), or usual care plus other interventions (n=1,639), and were then followed up for 28 days. The primary endpoints were time to first self-reported recovery, and hospital admission or death related to COVID-19, within 28 days.
Researchers found that, compared with the usual care group, participants using inhaled budesonide recovered an estimated 2.94 days sooner, had a greater sense of wellbeing while recovering and, once recovered, more often remained well.
For the hospital admission or death outcome, the estimated rate was 6.8% in the budesonide group, compared with 8.8% in the usual care group; although this failed to meet the superiority threshold.
The authors said that this failure resulted from decrease in hospital admissions, which may have been a result of the UK vaccination programme and lockdown measures.
They added: “Overall, the consistency of these findings across both primary and secondary endpoints provides the strongest evidence thus far of an effective, safe, cheap and readily available treatment for COVID-19 in the community.”
According to the authors of the study, early on in the COVID-19 pandemic, the low prevalence of asthma and chronic obstructive pulmonary disease among people admitted to hospital with COVID-19 led to speculation that the inhaled corticosteroids used to treat these conditions might be protective.
However, until now, there have been no results reported from large effectiveness trials of inhaled budesonide for COVID-19.
READ MORE: Everything you need to know about the COVID-19 therapy trials