Shutterstock / Christian Bertrand
A device which allows people to pump out food from their stomach after every meal via a surgically placed tube has been approved for the management of obesity by the US Food and Drug Administration (FDA).
The AspireAssist obesity device has been approved for use in patients aged 22 and older who have a body mass index of 35 to 55 and who have failed to achieve and maintain weight loss through non-surgical means. The device should not be used for patients with eating disorders, and it is not intended to be used for short durations in those who are moderately overweight.
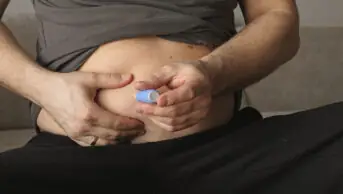
To place the device, surgeons make a small incision in the abdomen and endoscopically insert a tube into the stomach and through the incision. A disk-shaped port valve is left flush against the skin of the abdomen. Approximately 20 to 30 minutes after every meal the patient connects the valve to the device’s external connector and tubing to drain some of their stomach contents into a toilet. This takes approximately five to 10 minutes and removes about 30% of the calories consumed.
A clinical trial of 111 patients treated with AspireAssist and appropriate lifestyle therapy compared to 60 patients who received lifestyle therapy only, found that, after one year, patients using AspireAssist lost an average 12.1% of their total body weight compared to 3.6% for the control patients.
Adverse effects related to use of the device include indigestion, vomiting, constipation and diarrhoea. Risks associated with the surgical placing of the device or its removal include sore throat, bleeding, infection, sedation-related breathing problems, sores on the inside of the stomach, pneumonia, unintended puncture of the stomach and death. After device removal, there may be a risk of persistent fistula, an abnormal passageway between the stomach and the abdominal wall.
Patients using the device require frequent monitoring by a health care provider to shorten the tube as they lose weight and abdominal girth, so that the disk remains flush against their skin.