OJO IMAGES LTD / ALAMY STOCK PHOTO
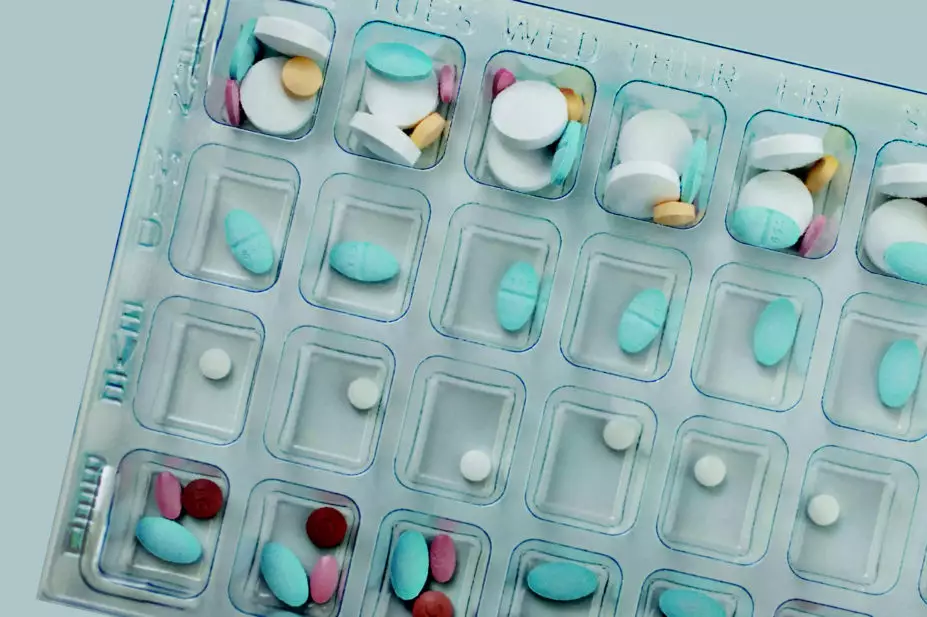
Monitored dosage systems (MDSs), also known as multi-compartment compliance aids, are medicine storage devices with compartments divided into days of the week and times of the day. Over the years, various terms have developed to describe these, such as ‘blister packs’, ‘monthly trays’, ‘medibox’ or ‘dosette boxes’. They come in many forms, including blister pack trays and rolls of pouches or sachets, and are intended to make it easier for patients to take their medicines and/or for carers to administer medicines.
However, it is unclear whether they provide a solution, or if they create problems for healthcare providers and patients.
For example, here is an all too familiar scenario with an MDS.
Case study: Mrs B
On a Friday afternoon, Mrs B, aged 83 years, is ready for discharge from hospital, where she has spent several days following an acute admission with community-acquired pneumonia, electrolyte imbalance and acute kidney injury.
Mrs B usually self-administers her medicines from a monthly MDS tray from her community pharmacy. The ward team have spent an afternoon earlier in the week contacting the busy community pharmacy and GP practice to convey the latest changes to Mrs B’s medicines to ensure her existing MDS is updated. Unfortunately, the community pharmacy had already prepared Mrs B’s monthly MDS tray, which was due this week, and this will now go to waste because it doesn’t contain her latest additions of short courses of slow K, co-amoxiclav, sando-phos and furosemide. And what about warfarin, which cannot be added to the MDS — will Mrs B remember to take it as well as the MDS medicines?
On discharge, the hospital pharmacy dispenses an initial supply of medicines to patients; however, the hospital pharmacy compliance aid robot only supplies medicines in a roll of pouches.
On demonstrating the system to Mrs B on the ward, it becomes clear that she does not have the dexterity to open these pouches and struggles to read the small label print. At one point, the tablets scatter across the floor as she attempts to open it.
In Mrs B’s case, discharge may be delayed and she will have temporary carers put in place to help her with her medicines management.
Disadvantages
Many medicines cannot be dispensed into an MDS owing to their unstable nature; for example, effervescent, dispersible or hygroscopic products, which are most sensitive to the effects of moisture, frequency of administration (e.g. Parkinson’s disease treatments), or their form (e.g. patches, topical creams and inhalers).
MDS devices are also unsuitable for ‘as required’ medication, such as analgesia, or medicines where the dose varies, such as warfarin. However, in these cases, it could cause confusion if patients were prescribed their medicines in both an MDS and original packaging.
Waste is also an issue when there are changes to regular medicines, such as in the case of Mrs B, who required a whole new MDS to be supplied mid-cycle.
The main concerns about MDS devices include the scope for human error, as well as the risk of destabilising medicines through exposure to moisture, light and contamination from other medicines or bacteria.
Use in care homes
In care homes, MDS devices should only be used where they support individual residents’ needs to enable self-administration and adherence. While many care providers believe that the use of an MDS improves the accuracy of medicines administration and provides a visual method of checking for staff members, there is no clear evidence that MDSs reduce medicines administration errors overall. Also, it is estimated that 40% of medicines in care homes cannot be dispensed within MDSs (e.g. topical products or unstable medicines); therefore, the use of MDSs results in parallel administration systems, making the process more complex.
Guidance from the National Institute for Health and Care Excellence (NICE) for adults receiving social care in the community states that MDS use should only be considered when an assessment by a healthcare professional has been carried out and a specific need identified to support medicines adherence.
Guidance issued by the Royal Pharmaceutical Society on better use of multi-compartment compliance aids states: “The use of original packs of medicines, supported by appropriate pharmaceutical care, should be the preferred intervention for the supply of medicines in the absence of a specific need for a multi-compartment aid in all settings.”
Best practice
There are many alternatives to MDSs out there to support an individual’s independence in continuing to take their medicines from original packs. An adherence assessment carried out by a healthcare professional, such as a pharmacist, is essential to determine the best option, alongside a patient-centred medicines review to ensure inappropriate polypharmacy is addressed and medicines are optimised.
Adherence solutions to consider include reminder charts and larger font labels. Alarms are also an option to remind patients to take their medicines, including a talking medication alarm clock. There are devices to help pop pills out of packaging and catch them in a container, thus reducing the risk of dropping tablets.
Most importantly, healthcare professionals should always speak to the patient, their family and any carers to find a solution that meets their overall health needs, helping them to remain independent where possible.


