Abstract
Three-dimensional (3D) printing is causing a paradigm shift in pharmaceuticals and clinical pharmacy practice, transitioning away from the traditional mass production of medicines towards tailored drug products that are personalised to each individual. The concept has the potential to provide benefits for patients, pharmacists and the pharmaceutical industry alike by enabling the on-demand design and production of flexible formulations with personalised dosages, shapes, sizes, drug release and multi-drug combinations. This is a turning point in the history of 3D printing technology in pharmaceuticals, requiring the engagement and support of healthcare staff, including pharmacists, doctors, nurses and pharmacy technicians, among others, to enable the widespread translation of the technology into clinical practice.
This article summarises the current state-of-the-art 3D printing methods and the major benefits and motivations for using 3D printing in pharmaceuticals, highlighting the critical role that healthcare staff play, and will continue to play, in the future integration of 3D printing into the pharmaceutical sector.
Key words: Additive manufacturing, clinical trials, digital health, patient-centric formulations, personalised medicine, pharmaceutical practice, precision medicine, technology, 3D printing.
Key points
- Three-dimensional (3D) printing is causing a shift away from traditional mass production towards personalised medicines;
- 3D printing could be integrated into various healthcare and resource-constrained settings;
- The technology can be used to create medicines that are tailored to a patient’s therapeutic requirements (e.g. dosage, drug combination and drug release profiles) and personal preferences (e.g. shape, size, texture and flavour);
- 3D printing offers numerous benefits to both clinical practice and the pharmaceutical industry alike, including decreased costs and expedited development time;
- Buy-in from healthcare professionals, including pharmacists, is vital to the integration of 3D printing into clinical settings.
Introduction
In recent years, the concept of personalised medicine has emerged, which involves the tailoring of medical treatment to each individual patient. Conventionally, medicines are mass manufactured in a limited number of discrete strengths, largely using technologies that were invented more than 200 years ago. Crucially, the selected dosing regimens represent the required dose for the safe and therapeutic effect in the ‘average’ patient[1]. However, it has become evident that one dose does not fit all; in the UK, up to 70% of patients do not gain efficacy from traditional mass manufacturing approaches, with 90% of drugs only working in 30–50% of the population and 7% of hospital admissions resulting from adverse drug reactions[2,3].
In 2015, the US Precisions Medicine Initiative was launched to understand how a patient’s genetics, environment, and lifestyle can help determine the best approach to prevent or treat disease[4]. Personalised medicine has also been placed at the forefront of the UK healthcare agenda. In 2016, the NHS published a report — ‘Improving outcomes through personalised medicine’ — and more recently the UK government published the ‘UK Genome Strategy 2020’ and the ‘Life Sciences Vision 2021’, both of which place personalised medicine as a priority within healthcare service delivery[5,6].
These initiatives are outlining how to move away from the ‘one-size-fits-all’ approach towards personalisation, requiring medication to be tailored to individuals, considering factors such as physiology, concurrent therapy, drug response, genetic makeup, disease state and other factors (e.g. sex, weight and age)[7]. Personalising treatments by tailoring medicines (e.g. combining more than one drug into the same tablet or selecting appropriate dosages) offers a plethora of opportunities including improved medication adherence, reduced adverse drug reactions and better therapeutic outcomes[8].
However, as the vision for personalised medicines becomes a reality, there is a crucial need to develop technologies that enable the transition away from the conventional large-scale production of fixed strength medicines towards creating flexible and personalised dosage forms and dose combinations on demand[9–11]. This transition can be enabled by 3D printing technologies[12]. Utilising a layer-by-layer production process, 3D printing can produce printlets (3D printed tablets) that are individualised to a patient’s therapeutic requirements (e.g. dosage, drug combination and drug release profiles) and personal preferences (e.g. shape, size, texture and flavour)[13–16]. To date, several studies have demonstrated the potential for 3D printing to create a wide variety of medicines, ranging from rapidly dissolving orodispersible formulations, controlled release preparations, gastro-retentive tablets, suppositories, minitablets, medical devices, as well as flexible multi-drug combinations (i.e. polyprintlets)[17–33].
Owing to the portable, compact and user-friendly nature of 3D printers, as well as the ability for 3D printing to manufacture medicines on demand, the technology could be easily integrated into healthcare settings, including hospital wards, in-patient pharmacies, specialist clinics and community pharmacies[15,34,35]. On demand dispensing in these scenarios could have significant benefits to clinical pharmacy practice, including improving medicines access and acceptability, reducing laborious and time-consuming extemporaneous preparation and accelerating discharge times[29]. Rapid production of one-off doses could be achieved in time or resource-constrained settings; for example, within hospital accident and emergency and acute medical care units, disaster areas, ambulances, military bases and in low- and middle-income countries[36].
Despite the advancements and benefits to both the pharmaceutical field and patients, several challenges remain, such as the need for clear regulatory guidance on the integration of novel point-of-care (POC) manufacturing technologies for the production of personalised medicines at the clinic, strategies for ensuring the quality and safety of formulations produced at the POC, and further engagement with healthcare professionals (including pharmacists) who will play a key role in implementing and managing this technology in the clinic.
The advancement of 3D printing, as well as other digital health technologies, is causing a paradigm shift in clinical pharmacy practice towards a vision of a novel and digitised treatment pathway that can adapt to the changing needs of each individual patient[37]. To date, one 3D printed drug product has been approved by the US Food and Drug Administration (FDA) for human use — levetiracetam in 2016[38].
In February 2021, FDA approval was granted for a clinical study using a 3D printed drug for patients with rheumatoid arthritis[39,40]. Then, in March 2021, the Medicines and Healthcare Products Regulatory Agency (MHRA) released a proposal for a new regulatory framework to enable the development of POC manufacturing and supply, including 3D printing technologies for personalised medicine production. The MHRA proposed that the assurance of safety for medicines manufactured at the POC would be based on established medicines development processes, such as non-clinical studies and clinical trials, but will be adapted to suit the requirements of multiple manufacture sites. These adaptions will support the development of new on-demand products, for example by avoiding the need for each POC site to be named on the marketing authorisation and to be individually inspected by the MHRA. Further details on the MHRA proposal can be accessed here[41].
Owing to the recent development and uptake of non-invasive drug and disease-monitoring strategies (e.g. smart wearable devices combined with artificial intelligence [AI]) and electronic prescriptions, 3D printing provides a unique platform that can produce medicines in response to changing situations and patient needs in a rapid, digital and decentralised manner[9]. Several studies have highlighted the potential for 3D printing technologies to be combined with AI for a multitude of benefits, including AI determining printability, as well as ensuring the quality and safety of the final printed drug product[42–46]. This concept could lead to a new era of digital pharmacy, enabling electronic prescriptions to be sent to a decentralised 3D printer location for real-time personalised medicine dispensing (see Figure 1). A wide range of stakeholders, including academic researchers, clinical pharmacists, doctors, biotech start-ups, large pharmaceutical companies and research funding bodies, are exploring this vision globally[12]. The Academy of Pharmaceutical Sciences (APS) organised the 2019 Additive Manufacturing Symposium to bring together academics, industry, biotech companies and regulators to discuss the concept of 3D printing pharmaceuticals, and in March 2021 set up the APS Emerging Technologies Focus Group that aims to advance technologies, such as 3D printing into practice.
This article will provide an overview of the main 3D printing methods used to produce pharmaceuticals and highlight the motivations and current cutting-edge applications of the technology in this sector. It will also summarise the remaining challenges to integration and discuss the critical role of healthcare staff as innovators in developing and integrating 3D printing into the pharmaceutical sector.
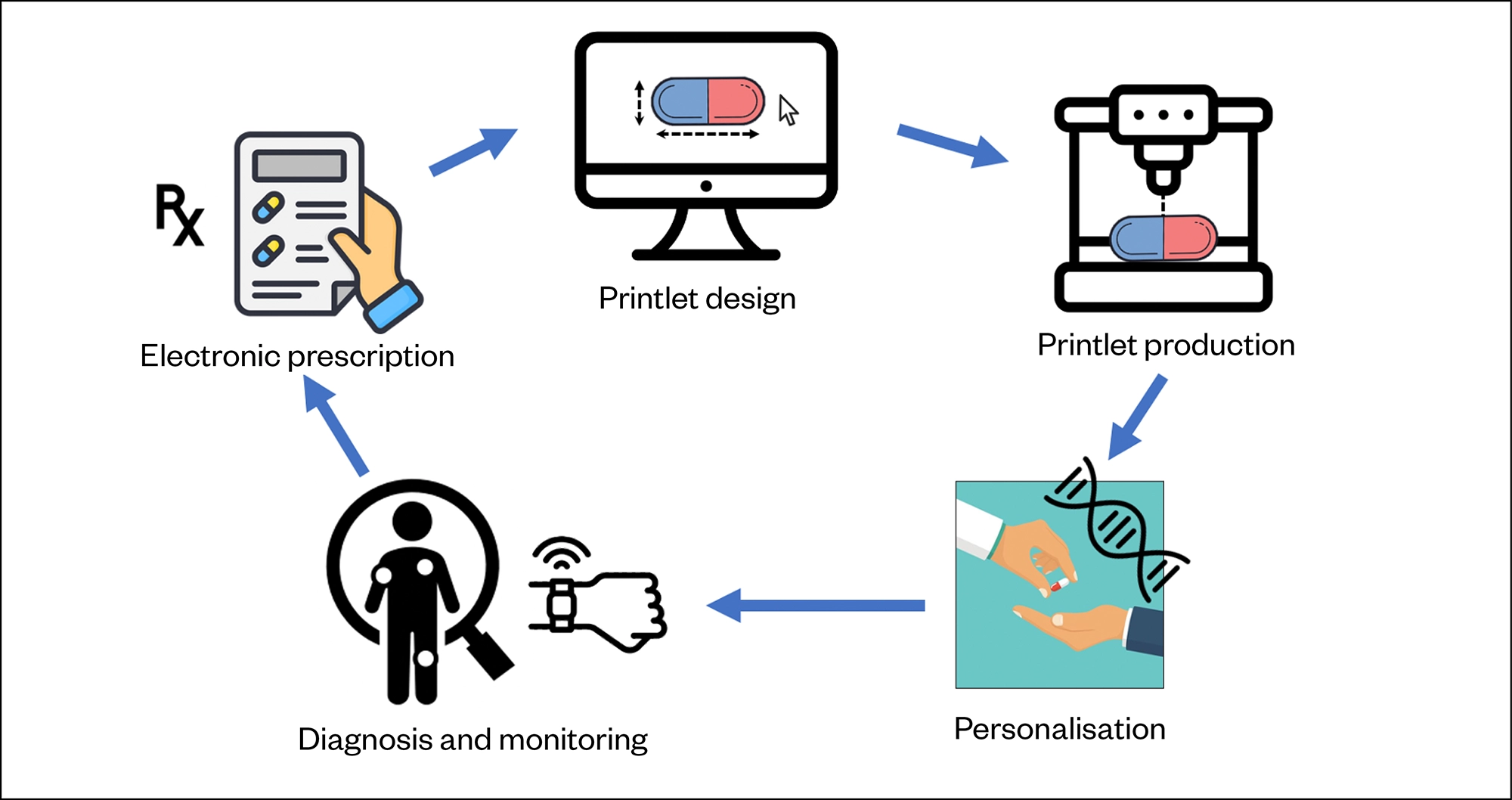
Types of pharmaceutical 3D printing systems
In 1986, 3D printing technology was developed and commercialised by Charles Hull; since then, several different 3D printing methods have been introduced[47–49]. The over-arching term ‘3D printing’ is now used to describe a wide range of printing technologies. Generally, all of these 3D printing technologies follow a common process for printlet production, described as the ‘3 Ds of 3D printing’ and provide a pathway for the future use and integration of this technology into clinical practice[15]:
Design: Using digital computer-aided design software, the pharmacist can design the formulation — for example, selecting the printlet geometry (shape and size) that can be targeted to the pre-clinical or clinical requirements. The designed formulation is then digitally transferred to the selected 3D printer;
Develop: Printlets are developed by inserting the required ‘ink’ cartridge (composed of a mix of drug and excipients) into the selected 3D printer. The most appropriate printing parameters are selected (e.g. resolution, temperature, printing time), which are typically based on the printer type, drug characteristics and desired outcomes;
Dispense: The 3D printer is then ready to automatically prepare the printed formulations layer by layer, which are then ready for ‘dispensing’ by the pharmacist.
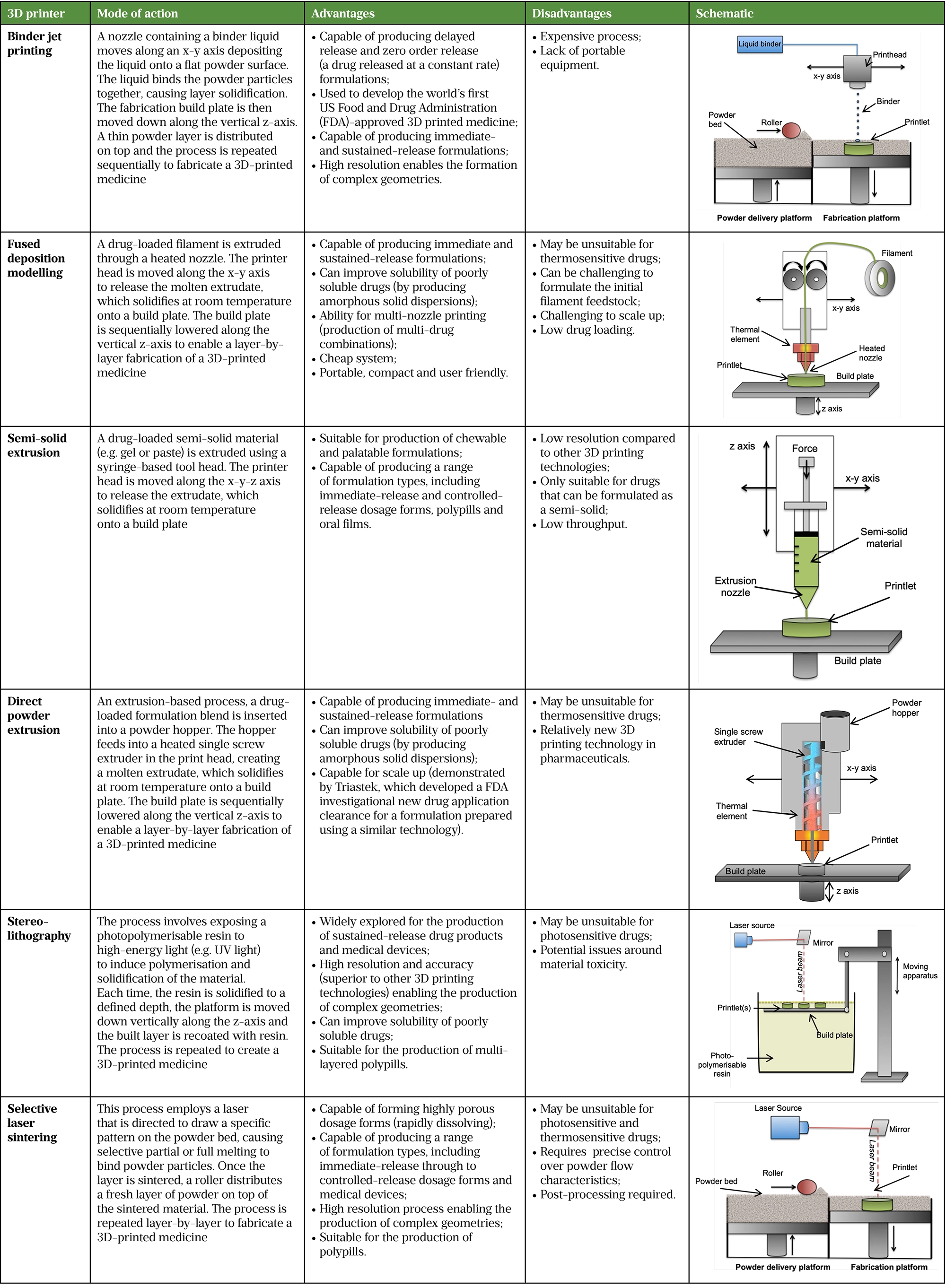
There are six main types of 3D printing methods explored in pharmaceuticals, described in Table 1.
To date, fused deposition modelling (FDM), selective laser sintering (SLS), stereolithography (SLA), binder jet (BJ) printing, direct powder extrusion (DPE) and semi-solid extrusion (SSE) have all been explored for the production of pharmaceuticals(50-54). Each technology comes with unique technical requirements and produces personalised drug products with a variety of characteristics — ranging from rapidly dissolving and orally disintegrating drug products to delayed and sustained release preparations. Table 1 details each technology, the types of drug products that can be produced and provides a schematic showcasing the technologies mode of action[38–64]. A full size version of this table can be found here.
Motivations for 3D printing medicines
The potential to personalise therapies in an automated and decentralised manner means that 3D printing provides various opportunities to patients, pharmacists, clinicians and the pharmaceutical industry alike.
Benefits to patients
A major benefit of 3D printing medicines is the ability to truly personalise a treatment based on a patient’s therapeutic or individual requirements. In the future, patients could be asked to select a formulation type from a catalogue; enabling the selection of characteristics such as formulation flavour, texture, colour, shape and size, leading to increased patient autonomy and engagement with treatment pathways and improved medicines adherence[15]. Enabling the production of medicines with exact dosages, or even containing flexible dosages of more than one drug to create a 3D printed polyprintlet, can also improve treatment efficacy while reducing the risk of adverse effects owing to inaccurate dosing[26,27].
Paediatrics
The ability to produce medicines with personalised dosage, flavour, shape and size can provide many benefits to paediatric populations, for whom conventional mass-produced formulations may not be suitable (e.g. owing to poor palatability or unsuitable dosages)[65,66]. Several studies have focused on producing child-acceptable formulations using 3D printing, including the production of chewable and even chocolate-based formulations[67,68].
For example, a 2018 study by Scoutaris et al. produced ‘candy-like’ formulations of several drugs, including indomethacin, that imitated Haribo Starmix® sweets using FDM[69]. However, while 3D printing can create formulations that are palatable to children, it is important to balance this against the risk of the formulations being too desirable and creating unintended risks to patient safety.
In 2020, Januskaite et al. evaluated the visual preferences of children aged 4–11 years of placebo printlets produced using four different 3D printing technologies, including digital light processing (DLP), the concept of which is similar to SLA, SLS, SSE and FDM[70]. Printlets were judged based on their familiarity, appearance, perceived taste and texture. Around 62% of children considered DLP printlets to be the most visually appealing, followed by SLS printlets, and FDM and SSE printlets. However, when the children were informed that the SSE printlet was chewable, the majority (79%) changed their original choice, which highlights children’s preference for chewable dosage forms[70].
In 2019, a world-first clinical study was carried out using a 3D printer to prepare personalised therapies in a hospital pharmacy setting[71,72]. This technology was integrated into the Clinic Hospital at University De Santiago de Compostela, Spain, to produce personalised medicines to treat children aged 3–16 years with maple syrup urine disease — a severe metabolic disease that stops the body from processing certain amino acids, causing a harmful build-up of substances in the blood and urine.
Chewable isoleucine printlets with six different flavours and colours, and four different dosages, were prepared using SSE, and researchers evaluated isoleucine blood levels as well as the acceptability of each formulation (see Figure 2). After six months of treatment, the 3D printed formulations demonstrated more desirable pharmacokinetic profiles of isoleucine and improved medicine acceptability among the participants, compared to standard isoleucine therapy. All of the formulations with different flavours and colours of the printlets were well accepted by patients, but flavour preference differed according to individuals[72]. Research is ongoing at Alder Hey Hospital in Liverpool for the production of personalised 3D printed hydrocortisone preparations for paediatrics[73].

Older people
3D printing may be beneficial to older populations or for those on complex dosing regimens where polypharmacy is common, leading to a high tablet burden. Studies have highlighted that polypharmacy can lead to non-adherence and confusion for patients, posing a risk of dosing errors[74].
There is value in combining multiple drugs, dosages and/or drug-release profiles into a single formulation that could improve medication adherence and reduce administration errors; however, conventional manufacturing processes do not currently support individualisation of ‘polypills’, producing only fixed-dose combinations. Owing to the flexibility and capabilities for accurate spatial distribution of drugs, 3D printing could be used to produce ‘polyprintlets’[75]. Several papers have demonstrated the production of polyprintlets using a range of technologies[75–78]. For example, in 2019 Robles-Martinez et al. prepared 3D printed polyprintlets using SLA, containing six different drugs (naproxen, aspirin, paracetamol, caffeine, chloramphenicol and prednisolone) separated into six different compartments, enabling the reduction of pill burden from six tablets to just one[26].
A 2015 study used FDM 3D printing to produce low-dose antihypertensive polypills containing atenolol, ramipril, pravastatin, aspirin and hydrochlorothiazide[22]. It should be noted that, while the printing of polypills is feasible using 3D printing, this will likely only be suitable for drugs with a low therapeutic dosage (microgram to milligram dose strength) to ensure the size of the formulation is a suitable size for administration.
In June 2021, FabRx (a spin-out biotech company from University College London [UCL]) and Gustave Roussy, a world-leading oncology hospital in Paris, announced a collaboration aiming to translate multi-drug 3D printed formulations into the clinic[46]. As part of this collaboration, personalised, multi-drug 3D printed dosage forms will be developed for the treatment of early-stage breast cancer patients, combining anticancer therapy with anti-side effect treatment into a single tablet. The printed medicines will be tested in a multi-centre clinical study to assess the effectiveness for improving acceptability, adherence and ultimately patient outcomes compared with the standard treatment regime.
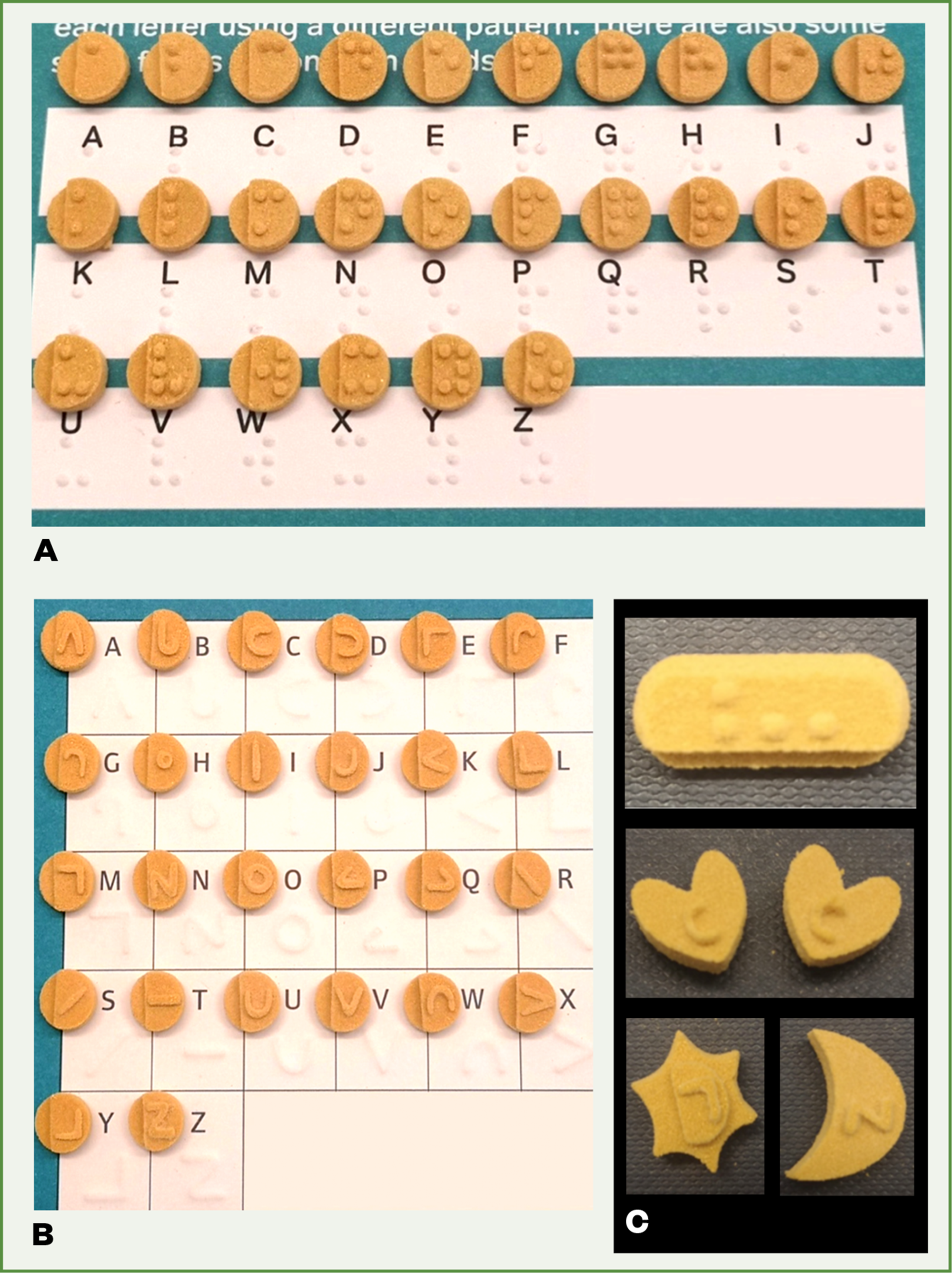
3D printing technologies may improve medicines independence. SLS has been employed to prepare orally disintegrating printlets with Braille and moon patterns on the surface of the dosage forms to enable visually impaired patients to identify medications (see Figure 3)[79]. The printlets were also produced in different shapes to offer additional information, such as the dosing regimen.
Benefits to clinical pharmacy practice
Owing to its portable, compact and user-friendly nature, 3D printers could be easily integrated into community and hospital pharmacy settings — FDM, SSE and DPE are particularly suited for this application. The main motivations for the integration of 3D printing technologies into clinical pharmacy practice is for pharmacists to have access to a highly flexible automatic compounding system, that can produce tailored dosage forms on demand based on the changing needs of the patient or situation. Such technology enables the production of printlets of any shape and size, as well as creating individual dosages for each patient, the ability to regulate the number of drugs in the formulation and to remove or replace individual components (e.g. active pharmaceutical ingredients or excipients) based on changing clinical requirements[80].
While it is unlikely that 3D printing will overtake the conventional mass manufacture of certain medicines, the capability for flexible manufacture is particularly useful in special patient populations and settings, as discussed above[15,81]. The majority of oral dosage forms are only commercially available as a single strength or formulation; pharmacists are therefore required to extemporaneously prepare bespoke formulations or to outsource the work to a specials manufacturing company[66]. Such practices are highly laborious, time consuming and costly, leading to significant delays and high expense for the development of patient-appropriate medicines. Patients and carers can often be asked to achieve a target dose of a particular medicine by manually splitting formulations, which is associated with an increased risk of inaccurate dosing, dose variation and dose-dumping for enteric coated tablets[82–85].
Studies have successfully explored the use of 3D printing to produce dose-flexible formulations containing drugs with narrow therapeutic indices, including warfarin, levothyroxine and tacrolimus[15,28,86,87]. Zheng et al. evaluated the potential for 3D printing to benefit extemporaneous manufacture by comparing the dose accuracy and content in 3D printed formulations compared to commercial tablets subdivided by pharmacists[88]. Compared with the manually split tablets, 3D printed spironolactone tablets were more accurate and safer[88].
3D printing offers an eco-friendly method of medicines manufacture; formulations can be produced with little or no drug and excipient wastage, reducing costs particularly for highly expensive drugs[15]. Medicines can also be produced on demand, reducing wastage of medicines liable to degradation upon storage, reducing the costs and environmental challenges that are associated with cold-chain storage and transport.
An on-site 3D printer to support extemporaneous preparation could improve medicine access for patients, reduce costs of manufacture and accelerate discharge times owing to reduced labour. One-off bolus doses could be rapidly produced in time or resource-constrained settings, such as within ambulances, disaster areas, hospital emergency departments or even for astronauts to produce their own medicines in space. Further work is required before integration into clinical practice can be attained, including the need for clear regulatory guidance on the integration of novel POC manufacturing technologies in the clinic, strategies for ensuring the quality and safety of formulations produced at the POC, and further engagement by clinicians and pharmacists who are important in implementing and managing this technology.
Benefits to the pharmaceutical industry
3D printing technologies pose many benefits to the pharmaceutical industry, especially within early phase drug development[10]. The time it takes from drug discovery to a marketed formulation is around 10–15 years, costing an average of £1.3bn[89]. There is an urgent need to reduce time and cost to market to expedite drug development timelines, made evident during the recent COVID-19 pandemic requiring rapid drug development and repurposing trials.
During pre-clinical and clinical formulation development, 3D printing could be used as a rapid prototyping tool to evaluate one-off or small batches of differing drug product iterations[10]. This rapid prototyping could speed up evaluating the impact of different formulation compositions on critical quality attributes (such as drug performance within in vitro and in vivo models). To date, 3D printed formulations have been tested in a wide variety of pre-clinical animal models[80,90,91]. As such, compared with laborious conventional manufacturing technologies, 3D printing could enable an earlier understanding of process and formulation variables, in turn allowing more rapid entry into first-in-human (FIH) clinical trials, reducing the time and cost of development. Moreover, 3D printing could be utilised throughout pre-clinical and early phase clinical trials to produce small batches of dose flexible drug products on demand to evaluate safety and efficacy[90].
3D printing could be used as an alternative manufacturing method in the pharmaceutical industry to produce mass customised or personalised medicines. From an economical perspective, it is likely that conventional methods of manufacture for high-volume and low-added value pharmaceuticals (such as tableting or encapsulation) will remain cost efficient in centralised facilities[10]. However, there is value in 3D printing formulations that require personalisation to improve therapeutic outcomes. Formulations could be mass customised to patient requirements using 3D printing at localised hubs, which is already being done in the healthcare industry; several major manufacturers use 3D printing to produce personalised hearing aids in a scaled up manner (around 1,000 devices per day), each being tailored in shape and size to the patient’s ear canal[92].
Pharmaceutical companies could use 3D printing to formulate medicines on demand in decentralised locations, such as within pharmacies, clinics or even the patient’s home. Furthermore, this could enable pharmaceutical companies to drive down transport costs and overall logistics expenses, in turn reducing the carbon footprint associated with transport and avoiding the need for energy-intensive storage conditions, such as cold-chain storage for thermosensitive drugs[10]. While the opportunities for 3D printing are still being explored, the integration of 3D printing into industry will require a shift in business model and approach will need to be carefully considered before 3D printing is taken up by pharma on a large scale.
The future of pharmacy: a roadmap to 3D printing integration
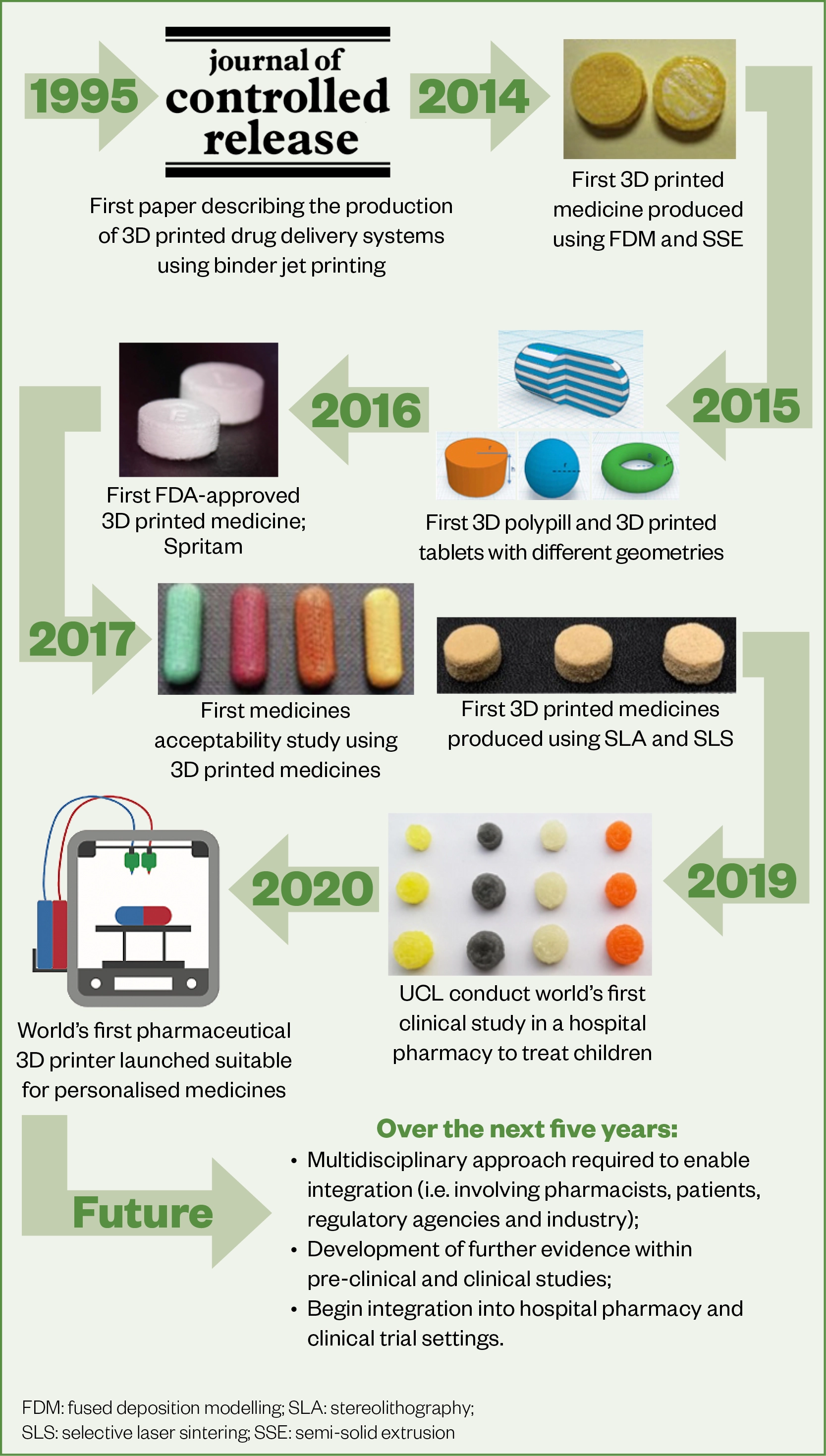
There are many published research papers demonstrating the potential and role of 3D printing technologies for medicines manufacture and patient care[93]. Figure 4 shows a timeline of 3D printing in pharmaceuticals and highlights the major milestones of this technology in the sector.
We are at a turning point, requiring buy-in from a number of key stakeholders, including researchers, regulators, clinicians, pharmacists and patients, to advance 3D printing technology for real-world benefit for pharmacists and patients. However, several challenges remain before widespread integration is possible in the pharmaceutical sector, including overcoming regulatory requirements, increasing the evidence base to demonstrate safety and efficacy and the further development of pharmaceutically-appropriate 3D printers and dosage forms that are widely accepted by pharmacists, clinicians and patients[80].
Major strides have been made towards overcoming these challenges. Previously, commercially available 3D printers were not standardised or fit for purpose to produce pharmaceutical products (i.e. not validated to good manufacturing practice [GMP]). In recent years, biotech companies have worked alongside regulatory agencies to develop pharmaceutical 3D printers that enable the production of printlets that are safe and effective for pharmaceutical use. One example is a pharmaceutical 3D printer that was developed by FabRx[94]. This is a multi-nozzle GMP printer suitable for medicines manufacture, developed in close communication with regulatory agencies, including the MHRA, and hospital end-users to create a system fit for this purpose.
Other pharmaceutical printers include Aprecia Pharmaceutical’s ZipDose technology, a scaled-up binder jet printing process to enable the mass manufacture of highly porous and rapidly orally-disintegrating drug products containing levetiracetam for the treatment of epilepsy[38].
Triastek has also recently launched a scaled-up melt extrusion system that enables the production of unique drug products for clinical trials[39]. Other companies, including DiHeSys, Vitae Industries and Craft Health, are also working towards producing GMP-compliant pharmaceutical printers, but no research has yet been published using these printing systems[95–97].
While the evidence base for 3D printing in pharmaceuticals is extensive, there is more work to be done for all parties to have confidence in the technology. There remain several regulatory and technical challenges, including strategies for ensuring the quality and safety of the medicines produced. Conventional medicine manufacture requires adherence to GMP and extensive testing requirements, including evaluating the risk of cross-contamination, weight uniformity and rigorous cleaning requirements[15]. However, such testing procedures are inherently costly, time-consuming and destructive; making them unsuitable for the evaluation of 3D printed medicines.
To overcome this challenge, work is underway to explore novel and real-time quality assurance mechanisms for 3D printed pharmaceuticals; for example, researchers have explored the use of real-time analytical technologies, such as near infrared and Raman spectroscopy, to ensure the quality and safety of formulations produced using 3D printers[98,99]. Others have suggested using ‘track and trace’ measures by including QR codes and data matrices on formulations that can be scanned using a smart phone device or barcode readers to ensure drug product quality and safety[100–102]. Others have highlighted the potential for use of a blockchain to track printed formulations and increase safety[103]. Additionally, as discussed above, several companies are developing GMP-compliant 3D printers that can be appropriately cleaned and validated to ensure printed drug product safety and quality. These innovative strategies will lead to a real-time assurance of 3D printed tablet quality and provide a strategy towards enabling integration of 3D printed technologies into the clinic.
Certain technical challenges remain before the technology can be widely used; there are currently a lack of suitable materials and excipients designed for the production of 3D printed medicines[104]. Furthermore, the use of 3D printing in decentralised locations will likely cause legal and ethical issues, such as challenges around patient data protection and security, risk of counterfeit production, as well as patient safety and liability considerations and the need for pharmacovigilance practices and policies to be in place to ensure adverse effects are appropriately identified and addressed.
To date, a limited number of studies have been performed in ‘real-world’ clinical settings involving patients to evaluate the acceptability of 3D-printed formulations and pharmacists as the end-users of the technologies. It is critical to move towards a multidisciplinary approach to 3D printing research, and crucially all major stakeholders (including pharmacists, clinicians, patients, and big pharma) are needed to discuss a way forward for this technology in the sector. Increased investment from research funders as well as support from regulatory agencies is also warranted.
The role of pharmacy in printing medicines
Pharmacists and pharmacy technicians working across sectors are paramount for enabling the adoption of 3D printing of pharmaceuticals and include those working on the front line as well as within academia, industry, regulatory agencies and government. As medicines experts, pharmacy staff can advise on the best strategy and route to market by defining how, when and where 3D printers could be best applied in pharmaceutical practice.
To date, globally, pharmacists have been the driving force behind 3D printing in pharmaceuticals, being the first profession to recognise the true potential of this technology for medicines production. The first studies that explored the use of FDM, SLA and SLS 3D printing for the production of medicines, in 2014, 2016, and 2017, respectively, were driven by academic pharmacists working at UCL School of Pharmacy, with other pharmacy schools around the world exploring a similar vision[105–107].
The vision for 3D printing in clinical practice will involve pharmacists and pharmacy technicians going back to their ‘roots’ as formulators; by designing and customising formulations based on the needs of specific patients and being experts on the use of 3D printers for the automatic extemporaneous preparation of formulations. One model could involve the 3D printer taking the form of a ‘Nespresso’ style system. This model would involve the drug-loaded cartridges being prefabricated at a manufacturing unit and the feedstock being quality and safety approved on-site, then distributed to local pharmacies for on-demand dispensing. Biotech companies are keen to make the systems user-friendly and safe, with an end-to-end tracking system and only allowing those who have been approved and appropriately trained to operate the system[94].
This route of pharmaceutical production is a novel and innovative solution to overcoming some commonplace problems in medicines manufacture, and requires a shift in mindset within the sector. Clinical pharmacists will lead the way in this area, working alongside university researchers, clinicians, pharmaceutical companies and clinical trial units to most effectively drive the translation of this technology.
The next generation of pharmacists, including MPharm students and preregistration pharmacists, must be trained on how to use this technology effectively and safely within practice. This can be done by educating staff on the appropriate situations to use 3D printing, providing technical oversight on the printing process and helping to ensure the quality of the formulations produced, as well as informing and engaging patients about these new types of medicines.
Conclusion
3D printing has the potential to revolutionise clinical pharmacy practice. It can transition conventional means of medicine mass manufacture towards the production of small batches of highly flexible and personalised dosage forms on-demand. This technology provides benefits for patients, pharmacists and the pharmaceutical industry alike by providing unique advantages such as making treatments safer and more effective. Healthcare professionals, including pharmacists, doctors, and nurses, are of paramount importance in enabling the integration of this technology and will be key to advising academics, the pharmaceutical industry and biotech companies on strategies to innovate the sector using 3D printing.
Acknowledgements and conflicts of interest
Abdul Basit is a co-founder and director of FabRx Ltd, a spin-out biotech company from University College London working to advance 3D printing as an automatic compounding platform for personalised medicines.
Event: 3D printing — enabling the future of healthcare
Royal Pharmaceutical Society, London, E1W 1AW
Pharmacy professionals, other clinicians, researchers, industry leaders and regulators are invited to this in-person event on 3 July 2025, from 10.30 to 18.30, aimed to explore the transformative potential of cutting-edge 3D printing technology, which is set to revolutionise the pharmaceutical landscape and deliver unparalleled patient care.
By attending this event, you will:
- Understand the fundamentals of 3D printing in pharmaceuticals and its impact on healthcare;
- Explore real-world applications of 3D printing, including patient-specific medicines and novel drug delivery systems;
- Gain insights into regulatory challenges and the evolving guidelines for implementing 3D printing in clinical and industrial settings;
- Identify key barriers to adoption, including quality assurance, cost-effectiveness, and manufacturing scalability;
- Learn from industry experts, researchers, and policymakers about the future of 3D printing in routine pharmacy practice;
- Contribute to the development of a strategic roadmap for safe and efficient integration of 3D printing in healthcare.
For more information and to register, see: https://events.rpharms.com/website/16267/
- 1Alomari M, Mohamed FH, Basit AW, et al. Personalised dosing: Printing a dose of one’s own medicine. International Journal of Pharmaceutics. 2015;494:568–77. doi:10.1016/j.ijpharm.2014.12.006
- 2Improving Outcomes Through Personalised Medicine. NHS England. 2016.https://www.england.nhs.uk/wp-content/uploads/2016/09/improving-outcomes-personalised-medicine.pdf (accessed Mar 2022).
- 3The Lancet. Personalised medicine in the UK. The Lancet. 2018;391:e1. doi:10.1016/s0140-6736(17)33261-0
- 4Sankar PL, Parker LS. The Precision Medicine Initiative’s All of Us Research Program: an agenda for research on its ethical, legal, and social issues. Genetics in Medicine. 2017;19:743–50. doi:10.1038/gim.2016.183
- 5Genome UK: The future of healthcare. Gov.uk. 2020.https://www.gov.uk/government/publications/genome-uk-the-future-of-healthcare/genome-uk-the-future-of-healthcare (accessed Mar 2022).
- 6Life Sciences Vision (HTML). Gov.uk. 2021.https://www.gov.uk/government/publications/life-sciences-vision/life-sciences-vision-html (accessed Mar 2022).
- 7Florence AT, Lee VHL. Personalised medicines: More tailored drugs, more tailored delivery. International Journal of Pharmaceutics. 2011;415:29–33. doi:10.1016/j.ijpharm.2011.04.047
- 8Wilsdon T, Edwards G, Lawlor R. The benefits of personalised medicine to patients, society and healthcare systems. EBE Biopharma. 2018.https://www.ebe-biopharma.eu/wp-content/uploads/2018/07/CRA-EBE-EFPIA-Benefits-of-PM-Final-Report-6-July-2018-STC.pdf (accessed May 2021).
- 9Awad A, Trenfield SJ, Gaisford S, et al. 3D printed medicines: A new branch of digital healthcare. International Journal of Pharmaceutics. 2018;548:586–96. doi:10.1016/j.ijpharm.2018.07.024
- 10Awad A, Trenfield SJ, Goyanes A, et al. Reshaping drug development using 3D printing. Drug Discovery Today. 2018;23:1547–55. doi:10.1016/j.drudis.2018.05.025
- 11Goyanes A, Scarpa M, Kamlow M, et al. Patient acceptability of 3D printed medicines. International Journal of Pharmaceutics. 2017;530:71–8. doi:10.1016/j.ijpharm.2017.07.064
- 12Trenfield SJ, Goyanes A, Gaisford S, et al. Editorial: Innovations in 2D and 3D printed pharmaceuticals. International Journal of Pharmaceutics. 2021;605:120839. doi:10.1016/j.ijpharm.2021.120839
- 13Goyanes A, Fina F, Martorana A, et al. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. International Journal of Pharmaceutics. 2017;527:21–30. doi:10.1016/j.ijpharm.2017.05.021
- 14Hamburg MA, Collins FS. The Path to Personalized Medicine. N Engl J Med. 2010;363:301–4. doi:10.1056/nejmp1006304
- 15Trenfield SJ, Awad A, Goyanes A, et al. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends in Pharmacological Sciences. 2018;39:440–51. doi:10.1016/j.tips.2018.02.006
- 16Öblom H, Zhang J, Pimparade M, et al. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis—Personalized Dosing and Drug Release. AAPS PharmSciTech. 2019;20. doi:10.1208/s12249-018-1233-7
- 17Gioumouxouzis CI, Tzimtzimis E, Katsamenis OL, et al. Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients. European Journal of Pharmaceutical Sciences. 2020;143:105176. doi:10.1016/j.ejps.2019.105176
- 18Reddy Dumpa N, Bandari S, A. Repka M. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics. 2020;12:52. doi:10.3390/pharmaceutics12010052
- 19Chatzitaki A-T, Tsongas K, Tzimtzimis EK, et al. 3D printing of patient-tailored SNEDDS-based suppositories of lidocaine. Journal of Drug Delivery Science and Technology. 2021;61:102292. doi:10.1016/j.jddst.2020.102292
- 20Krause J, Müller L, Sarwinska D, et al. 3D Printing of Mini Tablets for Pediatric Use. Pharmaceuticals. 2021;14:143. doi:10.3390/ph14020143
- 21Wang J, Zhang Y, Aghda NH, et al. Emerging 3D printing technologies for drug delivery devices: Current status and future perspective. Advanced Drug Delivery Reviews. 2021;174:294–316. doi:10.1016/j.addr.2021.04.019
- 22Khaled SA, Burley JC, Alexander MR, et al. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. Journal of Controlled Release. 2015;217:308–14. doi:10.1016/j.jconrel.2015.09.028
- 23Khaled SA, Burley JC, Alexander MR, et al. 3D printing of tablets containing multiple drugs with defined release profiles. International Journal of Pharmaceutics. 2015;494:643–50. doi:10.1016/j.ijpharm.2015.07.067
- 24Sadia M, Isreb A, Abbadi I, et al. From ‘fixed dose combinations’ to ‘a dynamic dose combiner’: 3D printed bi-layer antihypertensive tablets. European Journal of Pharmaceutical Sciences. 2018;123:484–94. doi:10.1016/j.ejps.2018.07.045
- 25Genina N, Boetker JP, Colombo S, et al. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: From drug product design to in vivo testing. Journal of Controlled Release. 2017;268:40–8. doi:10.1016/j.jconrel.2017.10.003
- 26Robles-Martinez P, Xu X, Trenfield SJ, et al. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics. 2019;11:274. doi:10.3390/pharmaceutics11060274
- 27Gioumouxouzis CI, Baklavaridis A, Katsamenis OL, et al. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. European Journal of Pharmaceutical Sciences. 2018;120:40–52. doi:10.1016/j.ejps.2018.04.020
- 28Seoane-Viaño I, Ong JJ, Luzardo-Álvarez A, et al. 3D printed tacrolimus suppositories for the treatment of ulcerative colitis. Asian Journal of Pharmaceutical Sciences. 2021;16:110–9. doi:10.1016/j.ajps.2020.06.003
- 29Yang Y, Xu Y, Wei S, et al. Oral preparations with tunable dissolution behavior based on selective laser sintering technique. International Journal of Pharmaceutics. 2021;593:120127. doi:10.1016/j.ijpharm.2020.120127
- 30Lin X, Fu H, Hou Z, et al. Three-dimensional printing of gastro-floating tablets using polyethylene glycol diacrylate-based photocurable printing material. International Journal of Pharmaceutics. 2021;603:120674. doi:10.1016/j.ijpharm.2021.120674
- 31Li Q, Guan X, Cui M, et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. International Journal of Pharmaceutics. 2018;535:325–32. doi:10.1016/j.ijpharm.2017.10.037
- 32Xu X, Goyanes A, Trenfield SJ, et al. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Materials Science and Engineering: C. 2021;120:111773. doi:10.1016/j.msec.2020.111773
- 33Liang K, Carmone S, Brambilla D, et al. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018;4. doi:10.1126/sciadv.aat2544
- 34Alhnan MA, Okwuosa TC, Sadia M, et al. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm Res. 2016;33:1817–32. doi:10.1007/s11095-016-1933-1
- 35Awad A, Trenfield SJ, Pollard TD, et al. Connected healthcare: Improving patient care using digital health technologies. Advanced Drug Delivery Reviews. 2021;178:113958. doi:10.1016/j.addr.2021.113958
- 36Norman J, Madurawe RD, Moore CMV, et al. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Advanced Drug Delivery Reviews. 2017;108:39–50. doi:10.1016/j.addr.2016.03.001
- 37Trenfield SJ, Awad A, McCoubrey LE, et al. Advancing pharmacy and healthcare with virtual digital technologies. Advanced Drug Delivery Reviews. 2022;182:114098. doi:10.1016/j.addr.2021.114098
- 38Spritam (levetiracetam); full prescribing information. Spritam. 2015.https://spritam.com/#/hcp/zipdose-technology/manufactured-using-3d-printing. (accessed Mar 2022).
- 39TRIASTEK RECEIVES FDA IND CLEARANCE FOR 3D PRINTED DRUG TO TREAT RHEUMATOID ARTHRITIS. 3D Printing Industry. 2021.https://3dprintingindustry.com/news/triastek-receives-fda-ind-clearance-for-3d-printed-drug-to-treat-rheumatoid-arthritis-184159/ (accessed Mar 2022).
- 40Zheng Y, Deng F, Wang B, et al. Melt extrusion deposition (MEDTM) 3D printing technology – A paradigm shift in design and development of modified release drug products. International Journal of Pharmaceutics. 2021;602:120639. doi:10.1016/j.ijpharm.2021.120639
- 41Consultation on Point of Care manufacturing. Gov.uk. 2021.https://www.gov.uk/government/consultations/point-of-care-consultation/consultation-on-point-of-care-manufacturing (accessed Mar 2022).
- 42Elbadawi M, Gustaffson T, Gaisford S, et al. 3D printing tablets: Predicting printability and drug dissolution from rheological data. International Journal of Pharmaceutics. 2020;590:119868. doi:10.1016/j.ijpharm.2020.119868
- 43Elbadawi M, McCoubrey LE, Gavins FKH, et al. Harnessing artificial intelligence for the next generation of 3D printed medicines. Advanced Drug Delivery Reviews. 2021;175:113805. doi:10.1016/j.addr.2021.05.015
- 44Elbadawi M, Muñiz Castro B, Gavins FKH, et al. M3DISEEN: A novel machine learning approach for predicting the 3D printability of medicines. International Journal of Pharmaceutics. 2020;590:119837. doi:10.1016/j.ijpharm.2020.119837
- 45Elbadawi M, McCoubrey LE, Gavins FKH, et al. Disrupting 3D printing of medicines with machine learning. Trends in Pharmacological Sciences. 2021;42:745–57. doi:10.1016/j.tips.2021.06.002
- 46FabRx And Gustave Roussy Enter Into An Agreement To Develop A Novel, Personalised, Multi-Drug Dosage Form For The Treatment Of Patients With Early-Stage Breast Cancer. FabRx. 2021.https://www.fabrx.co.uk/2021/06/25/fabrx-and-gustave-roussy-enter-into-an-agreement-to-develop-a-novel-personalised-multi-drug-dosage-form-for-the-treatment-of-patients-with-early-stage-breast-cancer (accessed Mar 2022).
- 47Apparatus for production of three dimensional objects by stereolithography. National Library of Medicine National Center for Biotechnology Information. 2022.https://pubchem.ncbi.nlm.nih.gov/patent/US-6027324-A (accessed Mar 2022).
- 48Durga Prasad Reddy R, Sharma V. Additive manufacturing in drug delivery applications: A review. International Journal of Pharmaceutics. 2020;589:119820. doi:10.1016/j.ijpharm.2020.119820
- 49Hemanth K, Hemamanjushree S, Abhinaya N, et al. 3D Printing: A review on technology, role in novel dosage forms and regulatory perspective. RESEARCH JOURNAL OF PHARMACY AND TECHNOLOGY. 2021;14:562–72. doi:10.5958/0974-360x.2021.00102.5
- 50Basit AW, Gaisford S, editors. 3D Printing of Pharmaceuticals. Springer International Publishing 2018. doi:10.1007/978-3-319-90755-0
- 51Awad A, Fina F, Goyanes A, et al. 3D printing: Principles and pharmaceutical applications of selective laser sintering. International Journal of Pharmaceutics. 2020;586:119594. doi:10.1016/j.ijpharm.2020.119594
- 52Awad A, Fina F, Goyanes A, et al. Advances in powder bed fusion 3D printing in drug delivery and healthcare. Advanced Drug Delivery Reviews. 2021;174:406–24. doi:10.1016/j.addr.2021.04.025
- 53Seoane-Viaño I, Januskaite P, Alvarez-Lorenzo C, et al. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. Journal of Controlled Release. 2021;332:367–89. doi:10.1016/j.jconrel.2021.02.027
- 54Xu X, Awad A, Robles-Martinez P, et al. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. Journal of Controlled Release. 2021;329:743–57. doi:10.1016/j.jconrel.2020.10.008
- 55Mostafaei A, Elliott AM, Barnes JE, et al. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Progress in Materials Science. 2021;119:100707. doi:10.1016/j.pmatsci.2020.100707
- 56Park BJ, Choi HJ, Moon SJ, et al. Pharmaceutical applications of 3D printing technology: current understanding and future perspectives. J. Pharm. Investig. 2018. doi:10.1007/s40005-018-00414-y
- 57Infanger S, Haemmerli A, Iliev S, et al. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. International Journal of Pharmaceutics. 2019;555:198–206. doi:10.1016/j.ijpharm.2018.11.048
- 58Dumpa N, Butreddy A, Wang H, et al. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. International Journal of Pharmaceutics. 2021;600:120501. doi:10.1016/j.ijpharm.2021.120501
- 59Melocchi A, Uboldi M, Cerea M, et al. A Graphical Review on the Escalation of Fused Deposition Modeling (FDM) 3D Printing in the Pharmaceutical Field. Journal of Pharmaceutical Sciences. 2020;109:2943–57. doi:10.1016/j.xphs.2020.07.011
- 60Sánchez-Guirales SA, Jurado N, Kara A, et al. Understanding Direct Powder Extrusion for Fabrication of 3D Printed Personalised Medicines: A Case Study for Nifedipine Minitablets. Pharmaceutics. 2021;13:1583. doi:10.3390/pharmaceutics13101583
- 61Goyanes A, Allahham N, Trenfield S, et al. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int J Pharm 2019;567:118471. doi:10.1016/j.ijpharm.2019.118471
- 62Chandekar A, Mishra D, Sharma S, et al. 3D Printing Technology: A New Milestone in the Development of Pharmaceuticals. Curr Pharm Des 2019;25:937–45. doi:10.2174/1381612825666190507115504
- 63Sen K, Mehta T, Sansare S, et al. Pharmaceutical applications of powder-based binder jet 3D printing process – A review. Advanced Drug Delivery Reviews. 2021;177:113943. doi:10.1016/j.addr.2021.113943
- 64Deshmane S, Kendre P, Mahajan H, et al. Stereolithography 3D printing technology in pharmaceuticals: a review. Drug Development and Industrial Pharmacy. 2021;:1–11. doi:10.1080/03639045.2021.1994990
- 65Tagami T, Ito E, Kida R, et al. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. International Journal of Pharmaceutics. 2021;594:120118. doi:10.1016/j.ijpharm.2020.120118
- 66Breitkreutz J, Boos J. Paediatric and geriatric drug delivery. Expert Opinion on Drug Delivery. 2006;4:37–45. doi:10.1517/17425247.4.1.37
- 67El Aita I, Rahman J, Breitkreutz J, et al. 3D-Printing with precise layer-wise dose adjustments for paediatric use via pressure-assisted microsyringe printing. European Journal of Pharmaceutics and Biopharmaceutics. 2020;157:59–65. doi:10.1016/j.ejpb.2020.09.012
- 68Karavasili C, Gkaragkounis A, Moschakis T, et al. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. European Journal of Pharmaceutical Sciences. 2020;147:105291. doi:10.1016/j.ejps.2020.105291
- 69Scoutaris N, Ross SA, Douroumis D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm Res. 2018;35. doi:10.1007/s11095-017-2284-2
- 70Januskaite P, Xu X, Ranmal SR, et al. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics. 2020;12:1100. doi:10.3390/pharmaceutics12111100
- 71FabRx completes a clinical study for the treatment of a rare metabolic disease in children using 3D printed dosage forms. FabRx. 2019.https://www.fabrx.co.uk/2019/04/11/fabrx-completes-a-clinical-study-for-the-treatment-of-a-rare-metabolic-disease-in-children-using-3d-printed-dosage-forms/ (accessed Mar 2022).
- 72Goyanes A, Madla CM, Umerji A, et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. International Journal of Pharmaceutics. 2019;567:118497. doi:10.1016/j.ijpharm.2019.118497
- 733D printing could give you a better pill to swallow. Mosaic Science. 2019.https://medium.com/mosaic-science/3d-printing-could-give-you-a-better-pill-to-swallow-d1114504f53f (accessed Mar 2022).
- 74Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opinion on Drug Safety. 2013;13:57–65. doi:10.1517/14740338.2013.827660
- 75Ghanizadeh Tabriz A, Nandi U, Hurt AP, et al. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment. International Journal of Pharmaceutics. 2021;593:120147. doi:10.1016/j.ijpharm.2020.120147
- 76Goyanes A, Wang J, Buanz A, et al. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharmaceutics. 2015;12:4077–84. doi:10.1021/acs.molpharmaceut.5b00510
- 77Rowe CW, Katstra WE, Palazzolo RD, et al. Multimechanism oral dosage forms fabricated by three dimensional printingTM. Journal of Controlled Release. 2000;66:11–7. doi:10.1016/s0168-3659(99)00224-2
- 78Goh WJ, Tan SX, Pastorin G, et al. 3D printing of four-in-one oral polypill with multiple release profiles for personalized delivery of caffeine and vitamin B analogues. International Journal of Pharmaceutics. 2021;598:120360. doi:10.1016/j.ijpharm.2021.120360
- 79Awad A, Yao A, Trenfield SJ, et al. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics. 2020;12:172. doi:10.3390/pharmaceutics12020172
- 80Seoane-Viaño I, Trenfield SJ, Basit AW, et al. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Advanced Drug Delivery Reviews. 2021;174:553–75. doi:10.1016/j.addr.2021.05.003
- 81Gioumouxouzis CI, Karavasili C, Fatouros DG. Recent advances in pharmaceutical dosage forms and devices using additive manufacturing technologies. Drug Discovery Today. 2019;24:636–43. doi:10.1016/j.drudis.2018.11.019
- 82Peek BT. Accuracy of Tablet Splitting by Elderly Patients. JAMA. 2002;288:451. doi:10.1001/jama.288.4.446
- 83Habib WA, Alanizi AS, Abdelhamid MM, et al. Accuracy of tablet splitting: Comparison study between hand splitting and tablet cutter. Saudi Pharmaceutical Journal. 2014;22:454–9. doi:10.1016/j.jsps.2013.12.014
- 84McDevitt J, Gurst A, Chen Y. Accuracy of tablet splitting. Pharmacotherapy 1998;18:193–7.https://www.ncbi.nlm.nih.gov/pubmed/9469693
- 85Hill S, Varker AS, Karlage K, et al. Analysis of Drug Content and Weight Uniformity for Half-Tablets of 6 Commonly Split Medications. JMCP. 2009;15:253–61. doi:10.18553/jmcp.2009.15.3.253
- 86Alomari M, Vuddanda PR, Trenfield SJ, et al. Printing T3 and T4 oral drug combinations as a novel strategy for hypothyroidism. International Journal of Pharmaceutics. 2018;549:363–9. doi:10.1016/j.ijpharm.2018.07.062
- 87Tian P, Yang F, Xu Y, et al. Oral disintegrating patient-tailored tablets of warfarin sodium produced by 3D printing. Drug Development and Industrial Pharmacy. 2018;44:1918–23. doi:10.1080/03639045.2018.1503291
- 88Zheng Z, Lv J, Yang W, et al. Preparation and application of subdivided tablets using 3D printing for precise hospital dispensing. European Journal of Pharmaceutical Sciences. 2020;149:105293. doi:10.1016/j.ejps.2020.105293
- 89Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020;323:844. doi:10.1001/jama.2020.1166
- 90Goyanes A, Fernández-Ferreiro A, Majeed A, et al. PET/CT imaging of 3D printed devices in the gastrointestinal tract of rodents. International Journal of Pharmaceutics. 2018;536:158–64. doi:10.1016/j.ijpharm.2017.11.055
- 91Shin S, Kim TH, Jeong SW, et al. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE. 2019;14:e0216875. doi:10.1371/journal.pone.0216875
- 92Ye M. The Impact of 3D Printing on the World Container Transport. TUDelft. 2015.https://repository.tudelft.nl/islandora/object/uuid%3Af16ee590-5804-4beb-b72c-a32346d0f175 (accessed Mar 2022).
- 93Trenfield SJ, Awad A, Madla CM, et al. Shaping the future: recent advances of 3D printing in drug delivery and healthcare. Expert Opinion on Drug Delivery. 2019;16:1081–94. doi:10.1080/17425247.2019.1660318
- 94FabRx’s pharmaceutical 3D printer for personalised medicines, M3DIMAKERTM, is now available! FabRx. 2020.https://www.fabrx.co.uk/2020/04/06/fabrxs-pharmaceutical-3d-printer-for-personalised-medicines-m3dimaker-is-now-available/ (accessed Mar 2022).
- 95DiHeSys – Digital Health Systems GmbH. DiHeSys – Digital Health Systems GmbH. https://www.digital-health-systems.com/kopie-von-start (accessed Mar 2022).
- 96Vitae Industries. Vitae Industries. https://www.vitaeindustries.com/ (accessed Mar 2022).
- 97Craft Health. Craft Health. https://www.crafthealth.me (accessed Mar 2022).
- 98Edinger M, Bar-Shalom D, Rantanen J, et al. Visualization and Non-Destructive Quantification of Inkjet-Printed Pharmaceuticals on Different Substrates Using Raman Spectroscopy and Raman Chemical Imaging. Pharm Res. 2017;34:1023–36. doi:10.1007/s11095-017-2126-2
- 99Trenfield SJ, Goyanes A, Telford R, et al. 3D printed drug products: Non-destructive dose verification using a rapid point-and-shoot approach. International Journal of Pharmaceutics. 2018;549:283–92. doi:10.1016/j.ijpharm.2018.08.002
- 100Trenfield SJ, Xian Tan H, Awad A, et al. Track-and-trace: Novel anti-counterfeit measures for 3D printed personalized drug products using smart material inks. International Journal of Pharmaceutics. 2019;567:118443. doi:10.1016/j.ijpharm.2019.06.034
- 101Edinger M, Bar-Shalom D, Sandler N, et al. QR encoded smart oral dosage forms by inkjet printing. International Journal of Pharmaceutics. 2018;536:138–45. doi:10.1016/j.ijpharm.2017.11.052
- 102You M, Lin M, Wang S, et al. Three-dimensional quick response code based on inkjet printing of upconversion fluorescent nanoparticles for drug anti-counterfeiting. Nanoscale. 2016;8:10096–104. doi:10.1039/c6nr01353h
- 103Nørfeldt L, Bøtker J, Edinger M, et al. Cryptopharmaceuticals: Increasing the Safety of Medication by a Blockchain of Pharmaceutical Products. Journal of Pharmaceutical Sciences. 2019;108:2838–41. doi:10.1016/j.xphs.2019.04.025
- 104Quodbach J, Bogdahn M, Breitkreutz J, et al. Quality of FDM 3D Printed Medicines for Pediatrics: Considerations for Formulation Development, Filament Extrusion, Printing Process and Printer Design. Ther Innov Regul Sci. 2021. doi:10.1007/s43441-021-00354-0
- 105Goyanes A, Buanz ABM, Basit AW, et al. Fused-filament 3D printing (3DP) for fabrication of tablets. International Journal of Pharmaceutics. 2014;476:88–92. doi:10.1016/j.ijpharm.2014.09.044
- 106Wang J, Goyanes A, Gaisford S, et al. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. International Journal of Pharmaceutics. 2016;503:207–12. doi:10.1016/j.ijpharm.2016.03.016
- 107Fina F, Goyanes A, Gaisford S, et al. Selective laser sintering (SLS) 3D printing of medicines. International Journal of Pharmaceutics. 2017;529:285–93. doi:10.1016/j.ijpharm.2017.06.082