Abstract
Aim
To undertake a preliminary evaluation of a community pharmacy scheme in which Parkinson’s disease patients could contact local specialist pharmacists about their Parkinson’s disease medicines.
Design
Longitudinal survey of patients registered in three primary care trusts.
Subjects and setting
145 patients with Parkinson’s disease were recruited into the study at baseline, and followed up six months later. The service was delivered within community pharmacy premises and through home visits in three primary care trust locations.
Outcome measures
The satisfaction with information about medication scales (SIMS), the medication adherence report scale (MARS). Quality of life was measured on the 39-item Parkinson’s disease questionnaire (PDQ-39). At follow up patients were also asked questions about their views of the service. Pharmacists also documented the problems they identified and actions they took as a result.
Results
Statistically significant improvements were found in the proportion of people indicating satisfaction with information on what the medicine does and on possible impact on sex life. Furthermore a significant improvement was found on the “potential problems of medication” summary score on the SIMS. During the period of the study self reported physical function improved on the PDQ-39. No changes were found on the MARS.
Conclusions
The results suggest improvements in patient satisfaction and potential effects on quality of life in terms of self-reported physical function, possibly as a result of the specialist pharmaceutical services. The data presented here could be used to suggest hypotheses for future studies and provide material for power and hence sample size calculations.
Parkinson’s disease is a progressive neurological disorder characterised by disability of movement, tremor, rigidity, slowness, postural disturbance and loss of balance. It is one of the commonest causes of disability in older people. It is estimated that at least 10,000 new cases are diagnosed in the UK each year. Average life expectancy, after diagnosis, is about 10 to 15 years. Only about 5 per cent of patients are aged under 40 years at diagnosis, and incidence increases rapidly with age, with most patients developing the initial symptoms between the ages of 50 and 70 years.
There is currently no cure for Parkinson’s disease, and treatment is directed towards alleviating symptoms. Typically, this is pharmaceutical. There are a number of drug treatments available, and their use depends on a variety of factors, including stage and severity of disease.1 Consequently, medication can seem complex and confusing to patients. The appropriate use of medicines is crucial to the management of Parkinson’s disease and the effects of these medicines are a key determinant of the quality of life for patients.2
Achieving the potential benefits of medication requires careful tailoring of the regimen to the needs of the individual.3,4 The recently published National Institute for Health and Clinical Excellence guideline for treatment of Parkinson’s disease notes that there is no single drug of choice for either early or late disease.
Two issues are particularly problematic: under-dosing and the experience of adverse effects. Regular monitoring and review is ideally required to assess for side effects and to confirm that the prescribed therapy is achieving the desired outcomes.5
Effective management of Parkinson’s disease is time consuming and complex, and may therefore present challenges to both primary care and neurology services. Community pharmacists are an integral part of the primary health care team and it has been suggested that better use can be made of their skills and knowledge for the successful provision of high quality, patient-centred services.6 Encouraging patient participation in management can lead to an improvement in patients’ knowledge and understanding of their disease and greater compliance with prescribed therapy.7
Under both the general medical services contract and the community pharmacy contract, health care professionals will need to work together more closely to demonstrate seamless patient care through development and implementation of enhanced services at a local level.8 Consequently, evidence is required to determine the uptake and impact of community pharmacy services.
The purpose of the pilot study reported in this paper was to undertake a preliminary evaluation of a community pharmacy scheme in which patients with Parkinson’s disease could contact local specialist pharmacists about their medicines. The aims were:
- To assess the feasibility of undertaking a project to evaluate the impact of community pharmacists on patients with Parkinson’s disease
- To gain data on which to base a sample size calculation for a larger survey or trial
- To provide a pragmatic preliminary evaluation of the project, in terms of levels of use, patients’ views of the service and whether there was any evidence it changed behaviour
Patients consulted community-based pharmacists who had received specialist training in the management of Parkinson’s disease. The training comprised a two-day workshop consisting of a clinical update, counselling and consultation skills and training in the practical organisation and delivery of the service. This latter section of the training was also attended by members of staff from the pharmacies involved.
The purpose of the specialised training was to enable pharmacists to consult patients with Parkinson’s disease confidently, elicit potential medicine-related problems and address them as appropriate. The training included the Centre for Postgraduate Pharmacy Education (CPPE) module on Parkinson’s disease, delivered by a local CPPE tutor and supplemented by input from local neurologists and Parkinson’s disease nurses. The intervention required pharmacists to work as part of the wider primary health care team, using their clinical knowledge, interpersonal skills and decision-making skills, in providing care that linked with that provided by GPs and neurologists. Pharmacists used a guide during each consultation which enabled them to record key issues related to medicine taking and Parkinson’s disease symptoms. Patients could consult the pharmacist at any time during the six-month evaluation, and were made aware of the availability of home visits. Pharmacists received remuneration for each consultation undertaken, up to a maximum of five per patient.
Methods
Patient recruitment
Three primary care trusts took part in the evaluation, working with 18 community pharmacists and their support staff. The PCTs (Brighton and Hove, Coventry, and St Helen’s) were selected based on their track record in delivering extended services via community pharmacists (eg, local pharmaceutical committee pilots), their ability to dedicate a local coordinator for a day a week to organise the service, and the willingness of the local neurology team to support the pilot. Pharmacists were selected locally by the PCT based on their commitment to training and service delivery and availability of a suitable consultation room within the pharmacy. Patients were recruited by one of three approaches:
- Letter of invitation from the local PCT
- Patients presenting a prescription for Parkinson’s disease medicines in participating pharmacies were asked to take part
- Information posters displayed in pharmacies or GP practices, or through branches of the Parkinson’s Disease Society, asked for volunteers
Patient pathway
Written consent was obtained from the patient and filed at the Health Services Research Unit (HSRU) in Oxford, which undertook the preliminary evaluation. Baseline questionnaires were then sent to patients for completion, together with a stamped addressed envelope, for return to the HSRU. After an initial consultation with the community pharmacist, patients were then free to return for further consultations within a six-month period. At follow up, questionnaires were mailed to evaluate the project.
The questionnaires
Three validated questionnaires, together with a set of supplemental questions, were administered at the start and after six months.
Satisfaction with information on medicines scale
The satisfaction with information on medicines scale9 (SIMS) is a 17-item questionnaire designed to assess the extent to which patients feel they have received enough information about prescribed medicines. The SIMS consists of 17 items derived from recommendations of the Association of the British Pharmaceutical Industry for the type of information that patients require in order to facilitate the safe self-management of medication.10 Each item refers to a particular aspect of taking medicines. Examples include “how to use your medicine” and “what you should do if you experience unwanted side effects”.
Participants were asked to rate the amount of information they received, using the following response scale: too much, about right, too little, none received, none needed. The responses are analysed at three levels:
- A detailed medicine information profile, obtained by examining the ratings for each individual item to identify individual types of information that patients feel they are lacking;
- A total satisfaction rating, obtained by summing the scores for each item. If the patient was satisfied that he or she received a particular aspect of medication information (ratings of “about right” or “none needed”), this is given a score of 1. If the patient was dissatisfied with the amount of information received (ratings of “too much”, “too little”, or “none received”), this scored 0. Summed scores ranged from 0 to 17 with high scores indicating a high degree of overall satisfaction with the amount of medication information received;
- Two subscale scores, identifying patients’ satisfaction with information about the action and use of medicines (items 1–9), and the potential problems of medication (items 10–17).
Medicine adherence report scale
The medicine adherence report scale7,11 (MARS) is a five-items scale that asks respondents to rate the frequency with which they engage in five aspects of non-adherent behaviour (eg, deciding to miss a dose, forgetting to take a dose), rated on a five-point scale (where 5 = never, 4 = rarely, 3 = sometimes, 2 = often and 1 = very often). Scores for each of the five items were summed to give a scale score ranging from 5 to 25, where higher scores indicate higher levels of reported adherence. The developers of MARS suggest that adherence can be expressed as a continuous scale.
Parkinson’s disease questionnaire
The Parkinson’s disease questionnaire (PDQ-39) is the most widely used disease-specific quality of life instrument used in studies of patients with Parkinson’s disease. Reviews have suggested that it is the most widely validated instrument and, in most instances, where quality of life is being measured it is likely to be the most appropriate.14,15 The PDQ-39 was developed on the basis of interviews and surveys of patients diagnosed with Parkinson’s disease. It measures eight dimensions of quality of life (mobility, activities of daily living, emotional well-being, stigma, social functioning, cognitions, communications and bodily pain). A method of taking missing data on the instrument into account has been suggested but was not used in this instance due to the small sample sizes.16
Project management and funding
The pilot was initiated by the European Parkinson’s Disease Association and organised by Medicines Partnership, a Department of Health programme that was based at the Royal Pharmaceutical Society. Training and day-to-day management of the pharmacists was provided by Pharmacy Alliance (now known as UniChem Professional Services). The project was co-funded by Pfizer and Medicines Partnership.
Ethics approval
Ethics approval for the study was granted by Oxfordshire Research Ethics Committee A on behalf of the Central Office for Research Ethics Committees (ref: 04/Q1604/22).
Results
Two hundred and thirty-one patients agreed to take part in the study and signed consent forms. Of these, 190 patients returned the baseline questionnaire, and of these, 145 returned the second (follow up) questionnaire. The analyses reported here are based on respondents who returned both. The mean age of patients was 69.88 years (SD 7.95; ranging from 45 to 91 years old). Eighty respondents (55.2 per cent) were male and 65 (44.8 per cent) female. Time since diagnosis ranged from two months to 29 years (median = five years).
Eight respondents (5.5 per cent) claimed never to have consulted the community pharmacist after the initial meeting. Most respondents (n=81; 55.8 per cent) consulted the specialist pharmacist once, twice or three times. However, a large number of respondents (33.1 per cent) did not answer this question. Participating pharmacists documented a total of 336 consultations over 12 months; 145 initial consultations and 191 follow-up consultations.
According to the information recorded, 55 per cent of the consultations were carried out in the pharmacy and 40 per cent were domiciliary.
Table 1 provides the detailed medication profile for the 17 areas measured by the SIMS. Statistically significant differences (McNemar’s test) in the proportion of people indicating satisfaction were found for information on what the medicine does (item 3) and possible impact on sex life (item 16). Three other items also indicated substantial, though not statistically significant, improvements, namely items how long one has to take the medicine, whether the medicine interferes with other medicines and whether or not the medicine causes drowsiness.
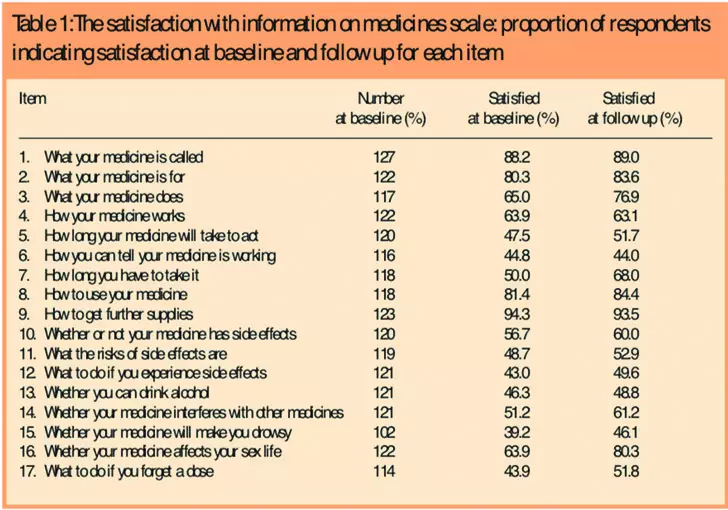
Table 2 provides summary statistics for the total satisfaction rating from the SIMS, together with the two sub-scale scores. All scores improved at follow up, but only the score for potential problems of medication reached statistical significance (chi-squared test, P<0.05).
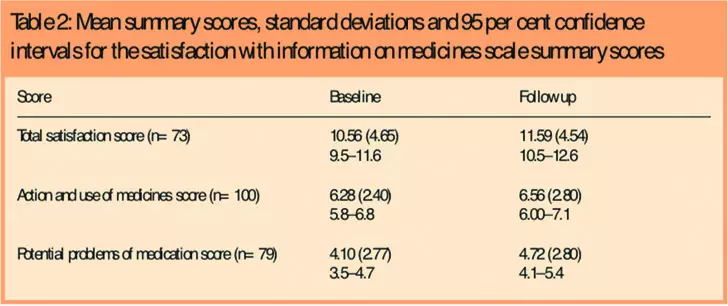
At baseline mean MARS scores were 23.17 (SD 2.02, n=122), and at follow up, 23.16 (SD 2.15, n=122), indicating a high level of compliance with medication advice at both baseline and follow up, but no change during the study.
The results of the PDQ-39 are reported in Table 3. Only the physical mobility dimension indicated statistically significant improvement between baseline and follow up (t=2.80, df=116, P<0.01) with a mean change of 3.87 (SD 14.97, 95 per cent CI 1.12–6.62).
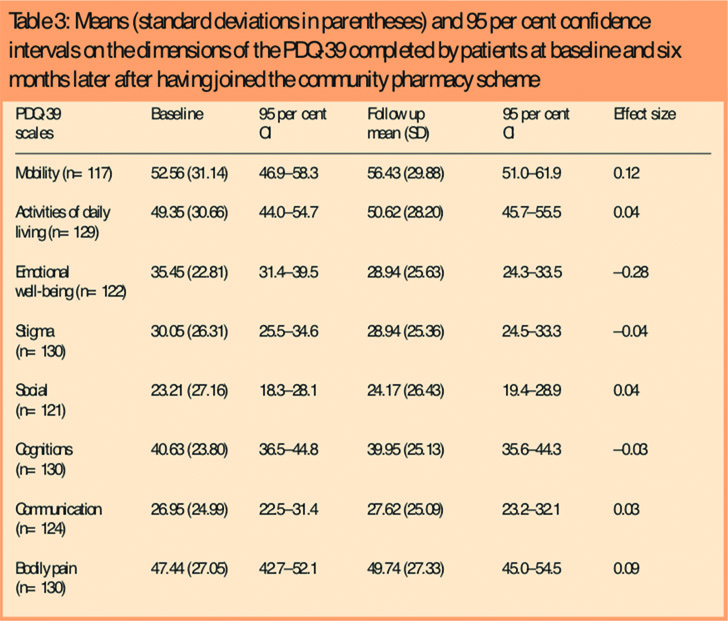
In the follow up questionnaire patients were asked what advice was provided and changes made to their treatment by the specialist pharmacists. Twenty-one patients (14.5 per cent) reported that the pharmacist recommended that they visit their GP, 12 (8.3 per cent) that they make arrangements to see their consultant and 29 (20.0 per cent) that they consult a nurse specialist. Pharmacists relatively rarely recommended changes in the type or strength of medicines used, but 30 respondents (21.5 per cent) reported they had been given advice about changing the time of taking their medicines.
At follow up, patients were asked about their attitudes to the initiative, and were generally positive about the experience (Panel 1).
Panel 1: Patients’ attitude to the initative
- 61 per cent said they knew more about their disease after involvement in the project
- 63 per cent claimed they knew more about their treatment
- 82 per cent claimed that the advice of the pharmacist was helpful
- 70 per cent claimed to have gained greater benefits from their drugs since taking part
- 87.5 per cent said the pharmacist was knowledgeable about Parkinson’s disease
- 90 per cent said the pharmacist listened to their concerns
- 87.5 per cent said they would recommend the service to others
Data recorded by the pharmacists during consultations and submitted to Pharmacy Alliance was also analysed. During the pilot, 531 medicines-related problems were identified and documented by participating pharmacists; 57 per cent (n=301) at first consultations and 43 per cent (n=230) at subsequent follow-up consultations. Table 4 illustrates the number and nature of problems identified.

On average, 1.5 problems were identified per consultation. The most frequently identified problems were uncontrolled symptoms, undesirable side effects and the need for a review of the dose or regimen.
Pharmacists reported that they made 596 interventions to address the problems identified, 74 per cent of which (n=443) were actioned in the pharmacy, via verbal or written advice or information. The remaining 26 per cent (n=153) required the involvement of the patient’s GP, nurse specialist or other health care professional. Table 5 illustrates the reasons pharmacists referred patients to another health care professional.
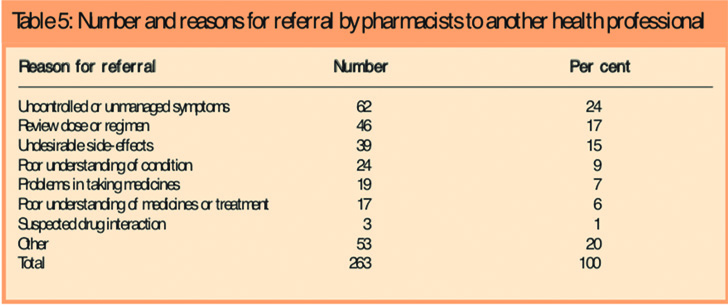
Discussion
The results of this specialist community pharmacy project are encouraging. The intervention was generally well received, and there is some evidence that it had a beneficial effect on satisfaction with information about medicines. Furthermore, during the six months, self-reported health status improved on the physical functioning domain of the PDQ-39. Spontaneous improvements in physical functioning in Parkinson’s disease are not commonly reported,17 and it is possible that better drug control, leading to improvements in physical functioning, was gained as a result of consultations with a specialist pharmacist.
A number of caveats must be mentioned in respect of the results of the study. First, the sample size is relatively small, and a larger controlled trial is needed to prove beyond reasonable doubt that interventions such as the one described in this paper are beneficial. Furthermore, recruitment was relatively slow, and because of the varied mechanisms adopted to attract patients into the study (ie, information posters, personal invitation in the pharmacy and letter from PCT) it is impossible to determine an overall take-up rate for the service. Second, the study was short. Greater benefits may accrue over time.
Finally, the apparent benefits of the interventions reported may be as a consequence of numerous statistical tests of significance, and any statistical adjustment for this (such as a Bonferroni correction) would lead to results suggesting no effect from the intervention. However, because this is an exploratory pilot study this seems inappropriate. Certainly, the results suggest avenues for further research, and indicate improvements in patient satisfaction and potential effects on quality of life as a result of the specialist pharmaceutical services. The data presented in this paper could be used to suggest hypotheses for future studies and provide material for power and hence sample size calculations.
This paper was accepted for publication on 30 March 2007.
About the authors
Geraldine Mynors, BA (Oxon), MBA, (Formerly) Head of Projects at the Medicines Partnership, Crispin Jenkinson, DPhil (Oxon), FFPH, is Professor of Health Services Research, Department of Public Health and Harris Manchester College, University of Oxford, Virginia MacNeill, PhD, FCIH, is research officer, Department of Public Health, University of Oxford, and Richard Balcon is NHS Services Manager at UniChem Professional Services Division
Correspondence to: Crispin Jenkinson at the Department of Public Health, University of Oxford, Old Road Campus, Headington, Oxford, OX3 7LF (e-mail crispin.jenkinson@dphpc.ox.ac.uk)
References
- Gray M, Baker M, Aujla M, Rajaei-Dehkordi Z, Sandhu P. A survey to assess patient, GP and pharmacist attitudes medicines management in people with Parkinson’s disease undertaken in the community pharmacy setting (survey pilot: phase 1). Pharmacetical Journal 2000;265 (Suppl):R25.
- Martinez-Martin P. An introduction to the concept of quality of life in Parkinson’s disease. Journal of Neurology 1998;245:S2–S6.
- Quinn N. Drug treatment of Parkinson’s disease. BMJ 1995; 310: 575–9.
- Thomas S, MacMahon D, Henry S. Moving and shaping the future. Commissioning Service for People with Parkinson’s Disease. Parkinson’s Disease Society UK, Primary Care Task Force 1999.
- National Institute for Health and Clinical Excellence. Parkinson’s disease. Diagnosis and management in primary and secondary care. NICE Clinical Guideline 35. London: National Institute of Health and Clinical Excellence, 2006.
- A vision for pharmacy in the new NHS. London: Department of Health, 2003.
- Erickson SR, Slaughter R, Halapy H. Pharmacists’ ability to influence outcomes of hypertension therapy. Pharmacotherapy 1997;17:140–7.
- Framework for a new community pharmacy contract. London: Department of Health, 2003
- Horne R, Hankins M, Jenkins R. The satisfaction with information about medicines Scale (SIMS): A new measurement tool for audit and research. Quality in Health Care 2001;10:135–40.
- Association of the British Pharmaceutical Industry. Patient information: advice on drafting of leaflets. London: ABPI, 1988.
- Horne R, Hankins M. The medical adherence report scale (MARS). Report published by the University of Brighton, 2003.
- Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well-being for individuals with Parkinson’s disease. Quality of Life Research 1995;4:241–8.
- Heffernan C, Jenkinson C. Measuring outcomes in neurological disorders — a review of disease specific health status instruments for three degenerative neurological conditions. Chronic Illness 2005;1:131–42.
- J, Ramaker C, van-Hilten JJ, Stiggelbout AM. Health related quality of life in Parkinson’s disease: a systematic review of disease specific instruments. Journal of Neurology Neurosurgery and Psychiatry 2002;72:241–8.
- Damiano AM, Snyder C, Strausser B, Willian M. A review of health-related quality-of-life concepts and measures for Parkinson’s disease. Quality of Life Research 1999;8:235–43.
- Jenkinson C, Heffernan C, Doll H, Fitzpatrick R. The Parkinson’s disease questionnaire: evidence for a method of imputing data. Age and Ageing 2006;35:497–502.
- Peto V, Jenkinson C, Fitzpatrick R. Determining minimally important differences for the Parkinson’s disease questionnaire (PDQ-39). Age and Ageing 2001;30:299–302.


