Abstract
Aim
To assess the feasibility of implementing a clinical teaching pharmacist programme to improve prescribing skills among newly qualified preregistration house officers (PRHOs).
Design
An observational pilot study.
Subjects and setting
13 medical PRHOs at Whipps Cross University Hospital, London.
Results
A 37.5% reduction (P=0.14) in prescribing errors after pharmacist intervention.
Conclusions
It is feasible for a teaching pharmacist to provide structured training to PRHOs in the clinical workplace. This pilot suggests that error rates are reduced by the intervention but a larger study is now required to confirm these findings.
Medication errors encompass any error in the process of ordering, dispensing, or administering a drug. Various members of the health care team may be involved, and such errors can lead to serious patient morbidity or mortality. Because drugs are used so frequently, the number of preventable injuries is substantial.1–4
Given that the prescription of a drug represents the most common health care intervention, prescribing errors are probably the most important source of medication errors and a major cause of adverse drug events.5,6
The evidence from the literature seems to suggest that there is no consensus on the definition of what constitutes an “error”, and different definitions such as “medication error”, “prescribing error”, or “adverse drug event (ADE)” seem to derive from the methods used to study their incidence. According to a recent review there is no standard denominator for use when expressing prescribing error rates in hospital inpatients.7 Because of wide variation in the definitions and methods used, it is difficult to make comparisons between different studies. It follows that the reported prescribing error rates in hospital inpatients vary widely, ranging from 0.3 per cent to 39.1 per cent of medicine orders written (ward drug charts), and from 1 per cent to 100 per cent of hospital admissions.7
A UK study8 developed the following definition of a prescribing error, on which our study was based: “A clinically meaningful prescribing error occurs when, as a result of a prescribing decision or prescription writing process, there is an unintentional significant (1) reduction in the probability of treatment being timely and effective or (2) increase in the risk of harm when compared with generally accepted practice.”
In the UK the prevention of medication errors to maximise patient safety is currently a key priority of the NHS. A Department of Health report addressed the issue of medication errors in the NHS, and was committed to reducing the number of serious errors involving prescribed drugs by 40 per cent by 2005. In line with this strategy, a further report from the Audit Commission10 recommended various strategies at a national and local level in order to tackle the problem of medication errors. A more recent government report11 reviewed the causes and frequency of medication errors and identified models of good practice to improve medication safety.
The positive impact of the clinical pharmacist interventions on prescribing quality and safety is well established.12–20 Pharmacists recommend initiation of therapy, monitor it and suggest modifications. This occurs on wards and in the dispensary. The percentage of medication charts that contain preventable medication errors detected by a pharmacist has been quoted in the range of 0.3 per cent to 0.8 per cent,21 and pharmacists have been found to take a drug history and write a drug chart better than junior doctors.14
The role of the pharmacist in helping optimise pharmaceutical care and reducing prescribing errors is part of the Government agenda.22,23 The Audit Commission report “A spoonful of sugar”10 recommends that pharmacists be integrated into the clinical team and work with doctors in order to anticipate medication errors and optimise drug therapies. This is crucial because most errors seem to occur during the prescribing stage mainly due to insufficient information at the time of prescribing.1
The report also recognised the problems faced by junior doctors: “Medication errors are more likely to happen when new doctors arrive to work in hospitals.”10 In line with that, it highlighted the need to provide formal induction of all new clinical staff and to continue training and competency assessment.
In the UK, hospital-based prescribing is heavily dependent on junior medical staff. Junior doctors are likely to make a substantial number of prescribing errors, particularly during the early induction weeks, possibly due to shortcomings in undergraduate and postgraduate training, as well as factors related to the working environment.24–27
It is generally acknowledged that there is currently little time for formal education of preregistration house officers (PRHOs) during the induction year, and a reasonably extensive induction/orientation period is necessary.6,10,28,29 There is the need for effective supervision of the PRHO with feedback on performance.
This study starts with the assumption that trainee doctors need to learn to prescribe medicines better rather than have this task taken from them, and the optimum setting for learning is the work environment in real life situations. The aim is to assess the feasibility of implementing a clinical teaching pharmacist programme to improve prescribing skills among newly qualified PRHOs. (This is optimally achieved during doctors’ ward rounds and by shadowing PRHOs’ prescribing whenever the opportunity arises.)
Methods
Setting
The study was conducted at Whipps Cross University Hospital, an 800-bed associate teaching hospital with a catchment population of 320,000 taken from the north-east of London and Essex borders.
The study took place on four medical wards normally staffed by four clinical pharmacists with between three and seven years of clinical experience. Two of the pharmacists only visit their wards once a day and spend an average of two hours per visit. They provide a basic pharmacy service which includes medicines supply for wards and patients, drug history taking and prescription monitoring. The other two pharmacists are ward-based. They usually visit their wards at least twice a day and spend a minimum of three hours on each ward. They are also available when needed via a paging system. The two ward-based pharmacists provide a more comprehensive clinical service, including extensive prescribing advice, discharge planning, patient counselling, junior doctor training, drug expenditure control and participation in senior physician-led ward rounds at least once a week.
All four wards admitted general medical emergencies but also had a degree of specialty triage covering cardiology, respiratory medicine, diabetes and endocrinology, gastroenterology, haematology and rheumatology.
Design
The study involved randomised allocation (through random number generation) of all 13 PRHOs in medicine within the trust to either the active (ward pharmacist) (n=6) or control (usual practice) (n=7) groups, with prospective data collection. Prescribing error data were collected before and after the intervention to allow comparative analysis. All PRHOs underwent a prescribing assessment at the end of the study period.
No sample size calculation was performed because the project was designed as a feasibility study with a small sample size available (only 13 medical PRHOs were allocated to the trust).
The prescribing of the 13 PRHOs was reviewed over a period of nine weeks, from August 2004 to October 2004. During the first two weeks of baseline data collection the four clinical pharmacists recorded errors from all prescriptions written by the 13 PRHOs, both inpatient and discharge medication prescriptions. Two data collection forms broadly resembling the existing trust pharmacist intervention forms were designed for the purpose, one for the inpatient medication charts and one for the “to take away” prescriptions (TTAs).
Three of the four pharmacists collecting data were blinded to the randomisation of the active and control groups. The principle investigator was aware of the randomisation as a necessity of the study design.
In order to minimise bias the study doctors were not informed of the timing of the data collection periods, although they were aware to which of the two groups they had been allocated, as this was impossible to “blind”. Usual practice of feedback to doctors of all the prescribing errors identified by the study pharmacists, during their routine recording activities, was maintained to safeguard patients.
The baseline period was immediately followed by a five-week intervention period, consisting of a senior clinical pharmacist accompanying the six junior doctors in the active group on their prescribing rounds. The ward rounds usually lasted about two hours and were led by consultants or senior registrars or senior house officers. The pharmacist only followed the teams on the study wards and was able to join the rounds on a twice daily basis within the 9am to 5pm period. In addition the pharmacist’s intervention included the following activities:
- Participation in physicians’ ward rounds, including consultant and trainee-led rounds, twice daily, on mornings, afternoons, or both
- Regular daily interactions with each doctor regarding prescribing issues on ward rounds and at other times when the opportunity arose
- Recording of all pharmacists’ interventions made during the day (grouped by doctor)
- Interactions with the other study pharmacists about any prescribing error that had been detected in order to bring it to the doctors’ attention
- Organisation and delivery of three structured teaching sessions focused on prescribing exercise workshops and critical appraisal of real examples of prescribing errors
- Compilation of a “Junior doctor prescribing guidelines handbook” derived from the question-and-answer component of the three formal teaching sessions
Three teaching sessions, one each week and aimed at both the active and the control groups (n=13), were also delivered by the pharmacist. The sessions focused mainly on prescribing exercise workshops and feedback from PRHOs on factors affecting their prescribing errors.
A further two-week data collection period immediately followed the pharmacist intervention period.
PRHO prescribing assessment
An independent assessment of the PRHOs’ prescribing skills was carried out at the end of the study period. All 13 PRHOs were required to answer correctly 12 questions in order to pass. The questions were based on a PRHO assessment programme developed at King’s College Hospital NHS Trust as part of the London deanery “modernising medical career” (MMC) assessment pilots.30
Definition and categorisation of prescribing errors
All types of inpatient charts were included except oncology and total parenteral nutrition charts. In general, the following were considered prescribing errors:
- Transcription errors
- Failure to take into account pharmaceutical issues (intravenous drug incompatibilities, drug interactions, contraindications, lack of monitoring of drug or patient parameters)
- Failure to communicate essential information (such as omissions in medication history taking)
- The use of drugs or doses inappropriate for the patient
Deviations from policies or protocols, or prescribing outside the hospital formulary guidelines, were not considered prescribing errors.
The errors were categorised into types as per the current trust pharmacist intervention form, which is derived from a national classification system (South Thames and North Thames regional pharmacy groups) as follows:
- Wrong drug prescribed
- Subtherapeutic dose
- Overdose
- Wrong dose frequency or dose time
- Medication history error
- Failure to document a previous averse drug reaction in a patient’s notes
- Failure to fill in an allergy box on a medication chart
- Inappropriate prescribing in renal or liver failure
- Drug contraindicated
- Duplication of therapy
- Unreasonable prolonged drug use
- Recommended inpatient medication not prescribed
- Incomplete or confusing prescribing
- Other (specify)
The errors were also categorised in terms of potential adverse risk to the patient into “severe”, “moderate-severe” or “moderate”. The classification, although relating to pharmacists’ interventions described in a previously validated study,31 was adapted for prescribing errors to help pharmacists grade the errors during their data collection. For practical reasons it was agreed that errors considered “minor” would not be considered during the data collection.
The prescribing errors that were graded as severe were assessed by an independent evaluator, a consultant in respiratory and general medicine. There was complete agreement between the evaluator and the study pharmacists.
Prescribing error rates: data analysis
The data were stored and analysed using the statistical package for social sciences SPSS for Windows, version 12. Baseline and post-intervention error rates (number of errors/ total of number of drugs prescribed) were compared between groups. The main outcome measure was the percentage change in prescribing error rates.
Once we obtained the mean percentage change in the pharmacy intervention group and the mean percentage change in the control group, the effect between the two groups was compared by performing the calculation of the 95 per cent confidence interval for each.
The test used to determine statistical significance was a t-test for paired data, which is performed using a “within each doctor analysis”. Two t-tests were performed, one each for active and control groups. This was to establish whether there had been a real change in prescribing errors after the pharmacist intervention.
When analysing each group of doctors as a whole, given the patients were independent events, a comparison of two proportions (ie, total prescribing error rates as a crude measure) was performed by using the Fisher’s exact test. This test ignores the fact that the same doctor was used before and after intervention, and it is appropriate for small numbers.
The Fisher’s test was also used to compare the two groups in relation to the final assessment (pass versus fail).
Ethics
All the study doctors were given an information sheet before the study started. Informed written consent was taken from the 13 doctors and all agreed to participate. All subjects were allocated a research code number and no attributable data were kept. All computerised information was registered under the Data Protection Act. The project was approved by the Redbridge and Waltham Forest Ethics Committee.
Results
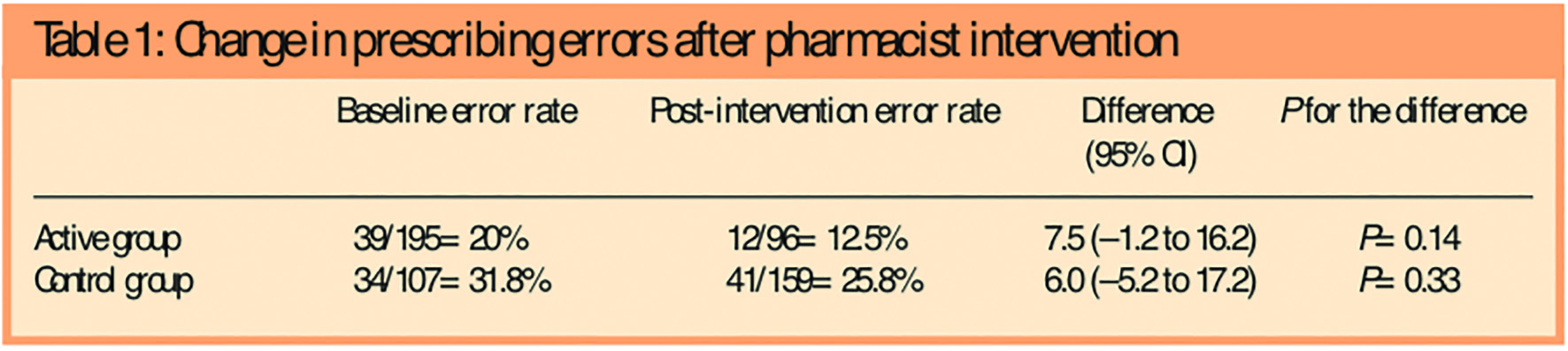
There was a 37.5 per cent reduction in total prescribing error rate in the active group after the pharmacist’s intervention (20 per cent v 12.5 per cent, P=0.14). In comparison the control group did not improve significantly during the whole study period: 18.9 per cent reduction in total prescription errors, P=0.33. (Table 1). The small sample meant that statistical significance was not reached.
After performing a “within-doctor-analysis”, the mean difference in prescribing error rates was obtained for each doctor based on the paired t-test. There was a greater variation in the active group, as the mean percentage change in prescribing error rate after the intervention was 7.2 per cent (P=0.07). In comparison the mean percentage change in prescribing error rate within the control group was 3.1 per cent (P=0.8).
The mean percentage change in TTA prescribing error rates in the active group was 10.1 per cent (P=0.03); the mean percentage change in drug chart prescribing error rates was 12.6 per cent (P=0.02). Both changes were statistically significant. In comparison, the mean percentage change in TTA prescribing error rates in the control group after the intervention was 4.2 per cent, (P= 0.9), and the mean percentage change in drug chart prescribing error rates was –13.9 per cent (P=0.25).
A total of 73 moderate and severe errors within the two groups were recorded during the baseline period, and 53 moderate and severe errors within the two groups were recorded during the post-intervention period.
After the pharmacist intervention, the active group made a lower percentage of moderate and severe errors as compared to the baseline period and to the control group. Conversely, the overall percentage of moderate and severe errors did not vary significantly within the control group.
During the post-intervention period, the active group made errors related to only five categories (fewer compared with the baseline
period). In comparison, the performance of the control group did not really differ from the baseline period as they made errors across all categories.
PRHO prescribing assessment
Only one control doctor did not perform the test. The active group performed better in comparison with the control group, as two PRHOs out of six passed the test (no PRHO passed in the control group) and the total mark score was higher (61 correct answers out of a possible 72 v 55/72). Although this difference was not statistically significant (P>0.1) possibly due to the small sample, the test strengthened the study results by showing that the PRHOs receiving the pharmacist intervention had a better pass rate and a higher test score.
Discussion
Prescribing errors in hospitals constitute a serious problem in the UK. It is generally acknowledged that junior doctors during their preregistration year are likely to make more errors, particularly during the early induction weeks. The main aim of this study was to assess the feasibility of implementing a clinical teaching pharmacist programme to improve prescribing skills among newly qualified PRHOs.
The study was designed as a pilot in order to test the feasibility of various methodologies and to examine prescribing trends. It proved possible for a single ward pharmacist to provide regular feedback and teaching to all PRHOs included in the intervention. The activity was well received by doctors who appreciated the nature of the work-based real time teaching. The error rates fell in the active group following the intervention. These PRHOs also scored more highly in the prescribing test. The small subject numbers meant that statistical significance was not reached.
The study has a number of limitations. Due to the nature of the unselected medical emergency cases admitted to the four wards it was not possible to control for case mix. Nevertheless all PRHOs rotated patient responsibilities between the four wards during the study period, thus minimising potential bias relating to error prevalence to a particular drug or group of drugs.
The principal investigator was one of the four study pharmacists, who performed both intervention and data collection in an attempt to ensure continuity and reliability. Practical reasons such as staff shortage contributed to this decision, although the potential for bias was fully taken into consideration.
Nevertheless it was thought that the independent contribution of the three study pharmacists in recording prescribing errors, as well as the independent PRHO prescribing assessment performed at the end of the study period, would minimise bias.
Given the nature of the study design, it was not possible for the clinical pharmacist to provide a standard number and similar quality of interventions to each PRHO during the intervention period; however the three teaching sessions were thought to optimise consistency in training.
It has been suggested that with the implementation of the foundation programme in August 2005 (“Modernising medical careers) the current discrepancies between undergraduate education and professional practice will be partly or totally eliminated. The two-year national programme will be based on the general training required to form the bridge between medical school and specialist/general practice training. Its learning objectives will be specific and focused on demonstration of clinical skills as well as general competencies such as clinical governance, risk management, infection control, team working, communication and IT skills, and the use of evidence and data.
Nevertheless the literature to date seems to suggest that trainee doctors need to learn to prescribe better rather than have this task taken away from them. A lot of junior doctors learn to prescribe from clinical pharmacists, not just in terms of the drugs but also how to prescribe safely and accurately, how to approach prescribing in a systematic way and how to use information sources.
Prescribing needs to be strengthened in undergraduate medical education and doctors need support in the foundation years 1 and 2. The results support the role of pharmacists and joint working. This has implications for undergraduate courses and also for the training of pharmacists at postgraduate level. There is the need to develop a joint training approach for prescribing — possibly extended to all health professionals.
The novelty of the study was the provision of a clinical pharmacist for workplace-based prescribing advice activities in an attempt to mimic the real life experiences of everyday practice. This was combined with intermittent structured teaching sessions. To date no other study had explored the hypothesis that pharmacist, hospital-based, on-site education, with interventions made at the time of or as close to the time of prescribing, is more likely to impact on prescribing behaviour of PRHOs as compared with structured teaching programmes.
The choice of a clinical pharmacist in order to help enhance PRHO prescribing skills and reduce errors seemed to be appropriate in light of current Government initiatives, which are designed to reduce medication errors through the involvement of pharmacists from various sectors. These initiatives include medicines management schemes, enhanced hospital clinical pharmacy services and supplementary prescribing.
As for possible future developments, provided that the study findings are confirmed by a larger study, and provided that more funding becomes available, the sustainability of the pharmacist intervention can be ensured by creating new “rotational teaching pharmacists” posts, both on medical and surgical wards, and on the emergency admission units.
The rotational structure would ensure continuity and consistency of the intervention. The prescribing support and teaching roles can be expanded to include final-year medical students by providing them with some of the basic skills that medical schools may have failed to deliver.
Acknowledgements
We thank Allan Hackshaw and Victor Lawrence for their help with the statistics, Kamaljit Takhar and Melissa Fong for their help with data collection, and pharmacists Ching-Yee Ngan and Eunice Omisakin.
This paper was accepted for publication on 10 January 2007.
About the authors
Daniela Webbe, MSc, MRPharmS, is principal pharmacist medicines management at Whipps Cross University Hospital.
Soraya Dhillon, PhD, MRPharmS, is head of the University of Hertfordshire school of pharmacy.
Michael Roberts, MD, FRCP, is consultant physician at Whipps Cross University Hospital.
Correspondence to: Daniela Webbe, Pharmacy Department, Whipps Cross University Hospital, Leytonstone, London E11 1NR (e-mail daniela.webbe@whippsx.nhs.uk)
References
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA 1995;274:29–34.
- Lesar TS, Lomaestro BM, Pohl H. Medication-prescribing errors in a teaching hospital: a 9-yearexperience. Archives of Internal Medicine 1997;157:1569–76.
- Vincent C, Neale G, Woloshynowych M. Adverse events in British hospitals: preliminaryretrospective record review. BMJ 2001;322:517–19.
- Kanjanarat P, Winterstein AG, Johns TE, Hatton RC, Gonzalez-Rothi R, Segal R. Nature ofpreventable adverse drug events in hospitals: a literature review. American Journal of Health-System Pharmacy 2003;60:1750–9.
- Dean B, Schachter M, Vincent C, Barber N. Prescribing errors in hospital inpatients: theirincidence and clinical significance. Quality and Safety in Health Care 2002;11:340–4.
- Dobrzanski S, Hammond I, Khan G, Holdsworth H. The nature of hospital prescribing errors.British Journal of Clinical Governance 2002;7:187–93.
- Dean Franklin B, Vincent C, Schachter M, Barber N. The incidence of prescribing errors inhospital inpatients: An overview of the research methods. Drug Safety 2005;28:891–900.
- Dean B, Barber N, Schachter M. What is a prescribing error? Quality in Health Care2000;9:232–7.
- Department of Health. An organisation with a memory. London: Stationery Office; 2000.
- Audit Commission. A spoonful of sugar — improving medicines management in NHS hospitals.London: Audit Commission; 2001.
- Department of Health. Building a safer NHS for patients. Improving medication safety. London:Stationery Office; 2004.
- Dhillon S. Do clinical pharmacists really improve the quality of patient care? HospitalPharmacist 2001;8:118.
- Dodd C. Assessing pharmacy interventions at Salisbury Health Care NHS Trust. HospitalPharmacist 2003;10:451–6.
- McFadzean E, Isles C, Moffat J, Norrie J, Steward D. Is there a role for a prescribing pharmacistin preventing prescribing errors in a medical admission unit? Pharmaceutical Journal2003;270:896–9.
- Stubbs J, Haw C, Cahill C. Auditing prescribing errors in a psychiatric hospital. Are pharmacists’interventions effective? Hospital Pharmacits 2004;11:203–06.
- Kucukarslan SH, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reducepreventable adverse drug events in hospital general medicine units. Archives of Internal Medicine 2003;138:2014–18.
- Leape L, Cullen D, Clapp MD, Burdick E, Demonaco HJ, Erickson JI, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA 1999;282:267–70.
- Dale A, Copeland R, Barton R. Prescribing errors on medical wards and the impact of Clinical pharmacists. International Journal of Pharmacy Practice 2003;11:19–24.
- Dhillon S, Duggan CD. An evaluation of interventions of major significance in North Thames Region. Continuing Professional Development Pharmacy 2001;1:12–18.
- Allen LaPointe NM, Jollis JG. Medication errors in hospitalised cardiovascular patients. Archives of Internal Medicine 2003;163:1461–6.
- Blum KV, Abel SR, Urbanski CJ, Pierce JM. Medication error prevention by pharmacists. American Journal of Hospital Pharmacy 1988;45:1902–3.
- Department of Health. Pharmacy in the future: implementing the NHS plan. London: Stationery Office; 2000.
- Royal Pharmaceutical Society. Pharmacy in a new age: the new horizon. London. The Society; 1996.
- Calman KC, Donaldson M. The pre-registration house officer year: a critical incident study. Medical Education 1991;25:51–59.
- Goodfellow PB, Claydon P. Students sitting medical finals — ready to be house officers? Journal of the Royal Society of Medicine 2001;94:516–20.
- Jones A, McArdle PJ, O’Neill PA. How well prepared are graduates for the role of the pre-registration house officer? A comparison of new graduates and educational supervisors. Medical Education 2001;35:578–84.
- Rolfe S, Harper NJN. Ability of hospital doctors to calculate drug dosages. BMJ 1995;310:1173–4.
- Barber N, Rawlins M, Dean Franklin B. Reducing prescribing error: competence, control,and culture. Quality and Safety in Health Care 2003;12:29–32.
- Dean B, Schachter M, Vincent C, Barber N. Causes of prescribing errors in hospital inpatients: a prospective study. Lancet 2002;359:1373–8.
- Cavell G, Scutt G. PRHO prescribing skills assessment. London: King’s College Hospital NHS Trust Pharmacy Clinical Services; 2004.
- Overhage JM, Lukes A. Practical, reliable, comprehensive methods for characterising pharmacists’ clinical activities. American Journal of Health System Pharmacy 1999;56:2444–50.