Introduction
Medicines are the most common intervention in health care, and their safe and effective use depends on a number of factors. Notable among these is that people taking medicines need to have access to appropriate information.[1]
This includes information about how to take medicines safely and effectively, and about the possible benefits and harms that can result from taking a medicine.[2]
If they are properly informed, people can take part in decisions about their medicines, as proposed in the concordance model of medicine taking.[3]
Until the mid 1990s, most people in the UK received no written information about their medicines and relied largely on verbal information from health professionals, most of which is forgotten.[4]
There was a marked change in 1999, when a new EU directive came into force.[5]
This required a comprehensive information leaflet for the patient (written by the manufacturer according to prescribed content and format) to be included inside every medicine pack. This move was in tune with UK policy trends for patient empowerment,[6]
and was followed by an EU guideline on readability.[7]
However, this major and costly innovation to provide a patient package insert in every medicine pack was not tested in advance, and there was no wider evidence-base to suggest that it would be beneficial to patients (or evidence to disprove that it might have adverse effects).[8]
In 1999, three months after the directive came into force, we undertook a preliminary study of its implementation and impact in the UK.[9]
We found that 32 per cent of medicines dispensed had no leaflet available for supply to the patient. Questioning of people who had collected prescriptions from pharmacies and who had been supplied with a leaflet revealed that 17 per cent reported not noticing the leaflet supplied. Of those who said they had seen the leaflet, 40 per cent reported they had read at least some of the leaflet and 21 per cent said they had read all of the leaflet.
In this paper we revisit the situation four years after implementation of the directive to investigate whether the proportion of prescriptions with a leaflet available and the proportion of people reading the leaflets have changed. In this follow-up study we collected more detailed information, including whether people were taking a medicine for the first time or were repeat users.
Method
This was a prospective study based in two community pharmacies in Leeds. Consecutive adult patients were recruited as they collected their prescriptions and asked, using a standardised protocol, if they would take part in research to study the information given to patients with their medicines. The local NHS research ethics committee approved the study and the inclusion criteria were:
- Aged 16 years or over
- Collecting their own prescribed medicine
- Sufficient use of English for interview
- Available for telephone interview
Having recruited individual patients, a list of the medicines dispensed was obtained from the pharmacy computer system and we determined whether a leaflet had been supplied by reference to dispensary staff. For every participant who received more than one medicine with a leaflet, a random numbers table was used to select one medicine leaflet on which to question the patient during the telephone interview. If a patient received no leaflet with a medicine, they received a courtesy call only.
After four to seven days, patients were telephoned and an 11-item structured questionnaire was administered. Any patient not contacted after five attempts was regarded as lost to follow-up. All data and responses to the questionnaire were collated, coded and entered into SPSSv11.0 for Windows. Frequencies and cross-tabulations were used in SPSS to analyse the ordinal data, using chi-squared as a test of statistical significance.
Results
In total, 194 consecutive patients were approached, of whom 152 (78 per cent) were successfully recruited. The reasons for non-recruitment were people not collecting their own medicine, not available for telephone follow-up, insufficient English, and did not wish to take part.
The cohort included 93 female participants (61 per cent) and the median age was 50 years (range, 16–88).
Leaflet supply
In total 321 prescription items were supplied, median one per person (range, one to 13). Of these, 265 were supplied with a leaflet and 56 were not. Of the 56 items supplied without a leaflet, nine were not prescription medicines (dressings, nutritional supplements etc). This left 312 prescription medicines, of which 47 (15 per cent) did not have a leaflet available for supply. This compares with a figure of 32 per cent in 1999 (P<0.0001).
Of the 152 recruited patients, 22 received no leaflet with any of their medicines, leaving 130, of whom 18 were unavailable for interview. This gave a total of 112 patients (86 per cent of those supplied with a leaflet) who completed the telephone interview. For 42 (37.5 per cent) it was the first time they had taken the medicine and 70 (62.5 per cent) said they had used the medicine in the past.
Awareness and retention of leaflet
The data from the principal questions are given in Table 1, and show that of those supplied with a leaflet, 98 (88 per cent) noticed it and 90 (80 per cent) said they had kept it.
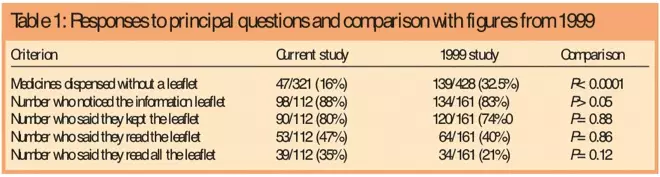
Table 1: Responses to principal questions and comparison with figures from 1999
Reading of leaflet
Fifty-three of 112 respondents (47 per cent) said they had read the leaflet supplied and 39/112 (35 per cent) said they had read the entire leaflet. The main reason for not reading the leaflet was that it had been read in the past (43/59 people; 73 per cent).
When asked which specific sections of the leaflet had been read, 51 of the 53 readers (96 per cent) reported reading the “side effects” section, 48 (91 per cent) “How and when to take it”, 45 (85 per cent) “What’s your medicine for”, 35 (66 per cent) “Things to do before taking” and 28 (53 per cent) “What’s in your medicine?”.
When asked when they first read the leaflet, 49/53 (92 per cent) said they had read the leaflet before using their medicine; when then asked how often they had read the leaflet since then, 34/53 (64 per cent) had not read since and 19/53 (37 per cent) had read at least once since then.
New versus old users
Those taking the medicine for first time were less likely to notice the leaflet (34/42; 81 per cent) than previous users (64/70; 91 per cent) (P=0.1). This compares with 79 per cent versus 89 per cent in the previous study. There was no difference in terms of keeping the leaflet 35/42 (83 per cent) versus 55/70 (79 per cent).
First-time users were more likely to read the leaflet, 30/42 (71 per cent) versus 23/70 (33 per cent) (P<0.02). In the previous study, 74 per cent of first-time users read it compared with 28 per cent of repeat users. First-timer users were more likely to have read all of the leaflet than repeat users 22/42 (52 per cent) versus 17/70 (24 per cent) (P=0.0025).
Discussion
Four years on from the implementation of the EU directive for mandatory medicines information leaflets, 15 per cent of medicines supplied through the study pharmacies had no leaflet available for the pharmacist to supply. This is a considerable improvement on the 32 per cent without a leaflet in 1999,[9]
but is still much less than the desired 100 per cent. Of those who received a leaflet, 12 per cent did not recall seeing it. This means that for up to a third of medicines, patients do not have the benefit of a leaflet and so had little or no information about the medicines they have been prescribed. The proportion in this study who did not notice the leaflet is higher for people taking the medicine for the first time. This is important, as Barber et al found that people using a new medicine have a substantial and sustained need for further information in the first 10 days and four weeks.[10]
However, the first time users who did notice the leaflet were more likely to read it than repeat users.
One of the reasons for supplying leaflets as a package insert is that it should guarantee that the patient will see it. This was not the case, with 12 per cent of people who did receive a leaflet with their medicine pack not recalling seeing it. This is a small improvement on the 17 per cent in 1999. Two possible reasons for not seeing the leaflet are that the carton was opened at the wrong end (leaving the leaflet tucked into the bottom of the carton). Alternatively, the ubiquitous nature of the package insert may lead to them being disregarded automatically.[11]
In terms of reading the leaflet, these results show that 47 per cent of patients said that they had read some of the leaflet compared with 40 per cent previously; and that 35 per cent said that they had read all of it compared with 21 per cent in 1999. Of those who had not read it, 73 per cent said that this was because they had read it in the past. It is not clear, therefore, how recently the leaflets had been read.
It is likely that patients’ responses to a leaflet depend on whether it had been supplied before. This is reflected here, where those taking the medicine for the first time were significantly more likely to read the leaflet (71 per cent versus 33 per cent).
Over 90 per cent of those who read the leaflet said that they had read it before using their medicine, which is encouraging, as much of the content of the leaflet is about how to use the medicine appropriately and important contraindications. When asked about particular sections of the leaflet that they had read, the most popular sections were “side effects”, “how and when to take it”, followed by “what your medicine is for”, “things to do before you take it” and “what’s in your medicine”, which mirrors previous research.[12]
Overall there has been a general increase in the visibility and level of reading of leaflets in the four years since the previous study. The findings of the current study concur with a recent National Audit Office survey which found that 30 per cent of people said they read all of a leaflet; but 8–12 per cent would never read any. Forty per cent thought there was too little information and 20 per cent would like to see more information on the likelihood of side effects.[13]
Another survey in 2003 for the Medicines Partnership found that 33 per cent of people surveyed had used a pack leaflet, but only 9 per cent of people said they would like to use leaflets more in the future (Shaw J, personal communication).
Two other parts of the world which have developed systems for distribution of medicine leaflets for patients are the US and Australia.[11] In the US, pharmacies print patient leaflets (not produced by manufacturers) at the time of first dispensing, which are less detailed than those required in Europe and not subject to legislation. A 2003 study showed that leaflets were supplied with 87 per cent of new prescriptions, but the quality of information varied greatly.[14]
In Australia, pharmacy computer leaflet generation is also employed, but manufacturers write the leaflets. In 2003, 79 per cent said they had read a leaflet in the past and 70 per cent of these said they had read all of it (unpublished observation, Koo, Aslani and Krass, National Medicines Symposium, Brisbane 2004).
There are a number of limitations to this study. It was undertaken in two pharmacies in one city, but there is no reason to believe the pharmacies or their patients were atypical. The positive response bias usually seen when people are asked about whether they have followed positive health behaviours means that the numbers reporting having read the leaflet are likely to be an overestimate. Also, recruiting people attending pharmacies will have resulted in under-recruitment of people unable to attend, notably the old and very old (people who take the most medicines).
Consumers want information about medicines but have different individual needs.[15]
It is also clear that there is general agreement that more information should be given[16]
and that standardised leaflets will never meet all of patients needs.[17]
Package insert leaflets, by their nature, are always general and standardised. The alternative option of computer-generation (as followed in the US and Australia) has the potential for flexibility, notably for individualisation and meeting the needs of people with special needs, such as those with sight impairment. The advantages of this method of delivery, led in 2003, to a wide-ranging UK collaboration which is developing web-based alternatives to package insert leaflets. Known as “Medicine guides”, they can be accessed via NHS Direct Online.[18]
In the meantime, package insert leaflets remain the most accessible information for most patients about their medicines. It is important that users are aware of the leaflet, particularly first time users. Health professionals, particularly pharmacists, are well placed to raise this awareness.
This paper has focused on supply and level of use of the leaflets with less emphasis on the content. Previous work suggests that neither the content not the design of current leaflets meet patient needs and they are not seen as important sources of medicines information[11],
[19]
This situation should improve, as a result of recent changes in EU pharmaceuticals legislation, which mean that from 2004, manufacturers will have to test their leaflets on the target population as part of the medicine licensing process.[20]
Acknowledgements
We thank the pharmacies and patients who took part in this study for their time and interest.
Declaration of interest
Theo Raynor and Peter Knapp are directors of LUTO Research Ltd, which provides a patient leaflet user testing service to the pharmaceutical industry.
David K. (Theo) Raynor, PhD, MRPharmS, is professor of pharmacy practice, medicines and their users, and Peter Knapp, PhD, RGN, is lecturer at the University of Leeds School of Healthcare. Alexandra Moody and Richard Young are medical students at the University of Leeds School of Medicine.
Correspondence to: Professor Raynor at School of Healthcare, University of Leeds, Leeds LS2 9JT (e-mail d.k.raynor@leeds.ac.uk)
References
[1] Coulter A. Evidence based patient information. BMJ 1998;317:225–6.
[2] Edwards A. Flexible rather than standardised approaches to communicating risks in health care. Quality and Safety in Health Care 2004; 13: 169-170
[3] Royal Pharmaceutical Society of Great Britain. From compliance to concordance. Achieving shared goals in medicine taking. London: The Society; 1997.
[4] Raynor DK. The influence of written information on patient knowledge and adherence to treatment. In: Myers L, Middence K (editors). Adherence to treatment in medical conditions. London: Harwood Academic; 1998.
[5] Council Directive 92/27/EEC of 31 March 1992 on the labelling of medicinal products for human use and on package leaflets. Official Journal 1992;(30 April 1992, No L113):8.
[6] Richards T, Partnerships with patients. BMJ 1998;316: 85–86.
[7] European Commission, Directorate-General III. A guideline on the readability of the label and package leaflet of medicinal products for human use. Brussels: European Commission; 1998.
[8] Raynor DK. PILs – on the wrong track? Pharmaceutical Journal 1998;260:55.
[9] Raynor DK, Knapp P. Do patients see, read and retain the new mandatory medicines information leaflets? Pharmaceutical Journal 2000;264:268–70.
[10] Barber N, Parsons J, Clifford S, Darracott R, Horne R. Patients problems with new medication for chronic conditions. Quality and Safety in Health Care 2004;13:172–5.
[11] Raynor DK, Savage I, Knapp P, Henley J. We are experts: people with asthma talk about their medicines information needs. Patient Education and Counselling 2004;53:167–74.
[12] Berry DC, Michas IC, Gillie T, Forster M. What do patients want to know about their medicines and what do doctors want to tell them? Psychology and Health 1997;12:467–80.
[13] National Audit Office. Safety, quality, efficacy: regulating medicines in the UK. London: NAO; 2003.
[14] Svarstad BL, Bultman DC, Mount JK, Tabak ER. Evaluation of written prescription information provided in community pharmacies: a study in eight states. Journal of the American Pharmacy Association 2003;43:383–93.
[15] Bessell TL, Anderson JN, Silagy CA, Samson LN, Hiller JE. Surfing, self-medicating and safety: buying non- prescription and complementary medicines via the internet. Quality and Safety in Health Care 2003;12:88–92.
[16] Barber N. Ensuring patients’ satisfaction with information about their medicines. Quality and Safety in Health Care 2001;10:130–1.
[17] Dickinson D, Raynor DK. Ask the patient – they may want to know more than you think. BMJ 2003;327:861
[18] All POMs to have medicine guides. Pharmaceutical Journal 2004;273:636.
[19] Consumers Association. Patient information: what’s the prognosis? London: The Association; 2003.
[20] Connelly D. User testing of PILs now mandatory. Pharmaceutical Journal 2005;275:12.


