Key points
- Individual clinical trials and meta-analyses show that progressive lowering of serum cholesterol levels produce greater relative risk reductions in cardiovascular disease.
- Individual clinical trials and meta-analyses document that the lowest cholesterol levels produce the greatest absolute reductions in risk.
- Several approaches (e.g. individual risk factors, global risk assessment and subclinical-atherosclerosis imaging) can be used to select patients for cholesterol-lowering drugs.
- Cholesterol-lowering drugs, especially statins, are generally safe but all drugs can have side effects that prevent their use in some patients.

Source: Zoonar GmbH / Alamy Stock Photo
Statins and other cholesterol-lowering drugs are increasingly being used for the primary prevention of atherosclerotic cardiovascular disease. In the micrograph image, cholesterol crystals.
Introduction
Statins lower serum cholesterol carried by atherogenic lipoproteins[1]
, such as low-density lipoprotein cholesterol (LDL-C) and very-low-density lipoprotein cholesterol (VLDL-C), which together constitute non-high-density lipoprotein cholesterol (non-HDL-C). Categories of LDL-C and non-HDL-C are listed in table 1. LDL-C is a term widely used in clinical practice, with ‘atherogenic cholesterol’ usually classified as either LDL-C or non-HDL-C. However, in this article, non-HDL-C is preferred over LDL-C because it includes all atherogenic cholesterol.
| Category | LDL cholesterol (mmol/L) | Non-HDL cholesterol (mmol/L) |
|---|---|---|
| Very high | >4.9 | >5.7 |
| High | 4.1–4.9 | 4.9–5.7 |
| Borderline high | 3.4–4.1 | 4.1–4.9 |
| Borderline low | 2.6–3.4 | 3.4–4.1 |
| Low | 1.8–2.6 | 2.6–3.4 |
| Very low | <1.8 | <2.6 |
Many randomised controlled trials (RCTs) show that statins effectively lower atherogenic cholesterol levels and reduce risk for atherosclerotic cardiovascular disease (ASCVD). Some investigators speculate that statins have benefits beyond cholesterol lowering; pleiotropic effects that remain to be proved.
On account of its high efficacy, statin therapy is virtually mandatory for most patients with established ASCVD (secondary prevention)[2],
[3]
but for the population as a whole, the greatest potential benefit lies in prevention of ASCVD in the first place (primary prevention). Statin use in primary prevention is the subject of great debate. Some investigators hold that statins are needed for most middle-aged and older people[4]
; others contend that statins should be restricted to those at highest risk for future ASCVD events[5]
. This article examines different points of view and attempts to provide a reasonable approach for use of statins in primary prevention. It is not strictly a comprehensive evidence-based review as these are available from major guideline committees in Europe, the UK and the United States and are referenced when appropriate throughout this article.
Sources
This article has attempted to develop reasonable recommendations for use of statins in primary prevention of cardiovascular disease by harmonising the results of statins, based on a review of the literature in the following areas: four major primary prevention trials with statins; two meta-analyses of secondary prevention trials showing the relationship between LDL cholesterol and risk for atherosclerotic cardiovascular disease; comparing the meta-analysis results with population and genetic epidemiological studies on the relation between LDL cholesterol and risk for atherosclerotic disease; comparing four recent and extensive evidence-based guidelines on treatment of blood cholesterol levels in primary prevention and safety and efficacy of statins in various populations under review. In addition, the paper draws insights from three recent clinical trials showing the efficacy of statins combined with other cholesterol-lowering drugs.
Discussion
Atherogenesis and primary prevention
A rationale for statin use in primary prevention is linked to the pathogenesis of atherosclerosis. This condition typically begins in young adulthood[6]
by cholesterol deposition in the arterial wall, as depicted in figure 1, which shows the major steps in atherogenesis. The first gross lesion is the fatty streak, which consists largely of cholesterol-enriched macrophages[7]
. Over time, the fatty streak grows and becomes encased in fibrous connective tissue called a fibrous plaque. Eventually, the plaque transforms into an advanced atheroma[8]
containing areas of calcification and structural degeneration that makes it vulnerable to rupture. When this happens, spillage of plaque contents into the arterial lumen precipitates thrombosis and the patient then experiences an acute cardiovascular syndrome such as myocardial infarction, stroke, or complications of peripheral vascular disease[9]
. It commonly takes 40–50 years from initiation of atherogenesis until clinical sequelae emerge.
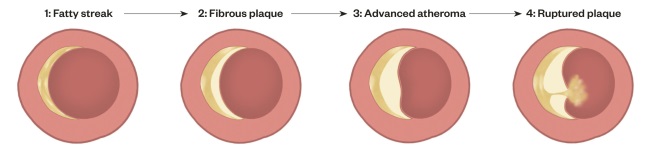
Figure 1. Stages of atherosclerosis
The first stage is called a fatty streak and is characterised by accumulation of cholesterol esters in macrophages in the intima. The second stage is a fibrous plaque where a fibrous connective tissue covers a lipid-rich core. In the third stage, the plaque is expanded (advanced plaque) and the volume of blood flow through the artery may be impaired. For example, in a coronary artery, an advanced atheroma can cause angina pectoris. In stage four, the plaque ruptures, releasing its contents into the lumen of the artery, precipitating a thrombosis and causing an acute cardiovascular event.
The aim of primary prevention is to slow progression at each stage of atherosclerosis development. A large body of evidence supports the view that atherogenic lipoprotein acts on every step of atherogenesis[10],
[11]
and reducing atherogenic cholesterol at any stage will slow the development of atherosclerosis. In the presence of elevated atherogenic lipoprotein, other so-called major risk factors accelerate atherogenesis and predispose to ASCVD events[6]
. Among these are smoking, hypertension, metabolic syndrome, and diabetes (see box 1). Age is commonly referred to as a risk factor, but age per se is not a cause but rather is a surrogate for subclinical atherosclerosis[12]
. Other emerging risk factors (see box 1) associate with ASCVD but whether they actually cause atherosclerosis or plaque rupture is uncertain[13],
[14]
. Some individuals are genetically susceptible to additional factors that enhance risk for ASCVD but are not well understood[15]
. Lifestyle factors such as a high-cholesterol diet, obesity, and physical inactivity either cause or worsen cardiovascular risk. Multiple risk factors commonly occur in a single individual, imparting a still higher risk for atherosclerotic disease. Even mild elevations of risk factors, especially when occurring together, can enhance risk. For this reason, investigators are increasingly asking whether statins should be employed as part of a public health strategy[4]
and the different answers to this question account for much of the controversy over the use of statins in primary prevention.
Box 1. Risk factors for atherosclerotic cardiovascular disease
Major risk factors:
- Smoking
- Hypertension
- Hypercholesterolaemia*
- Metabolic syndrome
- Diabetes
Emerging risk factors:
- Reduced high-density lipoprotein cholesterol**
- Elevated lipoprotein*
- Pro-inflammatory state***
- Pro-thrombic state****
- Pre-diabetes and insulin resistance
- Genetic variants*****
- Abdominal obesity and adipokines
- Homocysteine
*Includes elevated LDL-C and VLDL-C (non-HDL-C)
**Often included among major risk factors, but role in atherogenesis unclear
***Includes elevations in inflammatory cytokines and C-reactive protein
****Includes elevation in PAI-1 and fibrinogen
*****Includes a host of genetic variants associated with ASCVD
Cholesterol guidelines
US National Cholesterol Education Program
One long-standing guideline process in the United States has been the National Cholesterol Education Program (NCEP), which was underwritten by the National Heart, Lung and Blood Institute (NHLBI). Treatment recommendations were called Adult Treatment Panel (ATP) reports and were based on Framingham risk assessment[16]
. Three successive ATP reports were issued over a period of 20 years[13]
and included both primary and secondary prevention recommendations. Earlier ATP reports emphasised lifestyle therapies, but for high-risk patients, cholesterol-lowering drugs were permitted. In the third ATP report[13]
, however, drug treatment was liberalised and a threshold was developed for starting cholesterol-lowering drugs when an estimated 10-year risk for coronary heart disease (CHD) of >10% was reached. In patients with severe hypercholesterolaemia, cholesterol-lowering drugs were recommended regardless of estimated risk. LDL-C and non-HDL-C goals were also defined with the lowest goals set for highest risk patients. Statins were designated first-line drug therapy, but other drugs were allowed, if necessary, to achieve cholesterol goals. Globally, NCEP reports influenced subsequent guideline development.
European Cardiology Society and the European Atherosclerosis Society (ESC/EAS)
Guidelines of the ESC/EAS[17]
place high priority on lifestyle intervention for primary prevention and also base drug decisions on estimated risk by using SCORE (Systemic Coronary Risk Estimation)[18]
, with estimates based on total cardiovascular mortality. Statins are recommended when ten-year risk for cardiovascular mortality is >5%. This level corresponds roughly to an ASCVD-event rate of >15%. Goals for cholesterol-lowering therapy resemble those of NCEP reports[13]
.
Canadian Cardiovascular Society
In 2013, this society established goals for LDL-C for statin-treated patients[19]
. They proposed an LDL-C goal of <1.8 mmol/L both for primary and secondary prevention. In primary prevention, use of statins is recommended whenever 10% risk for CHD is >10% (or for ASCVD approximately 15%). Although statins are primary therapies, other non-statin drugs are permitted when considered necessary to attain the goals of therapy.
American College of Cardiology and the American Heart Association (ACC/AHA)ÂÂÂ
In 2013, the NCEP transferred cholesterol guidelines to the ACC/AHA. These organisations largely restricted recommendations to statins[20]
as RCT evidence for non-statin drugs was considered too weak for strong recommendations. Different statins and doses are classified according to the intensity of cholesterol lowering (table 2). The threshold for starting statins is a ten-year risk for ASCVD (i.e. CHD plus stroke) of >7.5%. This threshold corresponds to a ten-year risk for CHD of approximately 5%. In parallel, a new risk-assessment algorithm is used[21]
. For primary prevention, when estimated risk is over the treatment threshold, a high-intensity statin (e.g. atorvastatin 80mg) is recommended. A moderate-intensity statin is permitted if the high-intensity statin is not tolerated. No target goals for atherogenic cholesterol levels are specified.
| Drug | Low intensity | Moderate intensity | High intensity |
|---|---|---|---|
| 20–25% ↓ LDL-C | 30–45% ↓ LDL-C | > 45% ↓ LDL-C | |
| Lovastatin | 10mg | 40–80mg | |
| Pravastatin | 10mg | 40–80mg | |
| Simvastatin | 10mg | 20–40mg | |
| Fluvastatin | 40mg | 80mg | |
| Pitavastatin | 2–4mg | ||
| Atorvastatin | 5mg | 10–20mg | 40–80mg |
| Rosuvastatin | 5–10mg | 20mg | |
| Statin and ezetimide | Simvastatin 10mg (or other low-intensity statin) and 10mg ezetimibe | Simvastatin 40mg (or other moderate-intensity statin) and 10mg ezetimibe | |
| Statin and bile acid resin | Simvastatin 10mg (or other low-intensity statin) and resin (variable dose depending on resin) | Simvastatin 20–40mg (or other moderate-intensity statin) and resin (variable dose depending on resin) |
National Institute for Health Care Excellence (NICE)
Another guideline, widely used in the UK, is offered by NICE[22]
. It employs the QRISK algorithm to estimate ten-year risk for cardiovascular disease (CVD)[23]
. NICE recommends atorvastatin 20mg when the ten-year risk for CVD is >10%. NICE guidelines favour non-HDL-C over LDL-C as a target of treatment, but no goal is set for atherogenic cholesterol, other than noting that non-HDL-C levels ideally should be reduced by approximately 40%.
Evidence base for statin therapy
Three lines of evidence underlie recommendations for statin therapy:
Benefits of greater percentage of cholesterol reductions: ‘the more, the better’
The Cholesterol Clinical Trialists Collaboration[3]
carried out a meta-analysis from many statin RCTs and observed that for every 1mmol/L reduction in LDL-C, the risk for future ASCVD events decreases by approximately 20%. Presumably, the same relation holds for non-HDL-C. Also, for every 1% reduction in atherogenic cholesterol, risk for ASCVD is decreased by approximately 1%. A similar result was found in a meta-analysis of primary prevention trials[24]
. This relationship, which appears to be linear over a broad range of cholesterol levels (although it has not been adequately tested at very low cholesterol levels), suggests that the greater the percentage reduction of atherogenic cholesterol, the greater the reduction in risk will be. Under this paradigm, no lower goals for cholesterol levels are needed. The concept of ‘percentage reductions as targets’ influenced the development of recent cholesterol recommendations (e.g. 2013 ACC/AHA guidelines[20]
and the 2014 NICE report[22]
). For drug therapy in primary prevention, ACC/AHA recommend high-intensity statins (e.g. atorvastatin 80mg)[20]
that will, on average, reduce LDL-C by approximately 50%, and the NICE guidelines favour a fixed dose of atorvastatin 20mg, which, on average, will reduce non-HDL-C by around 40%[22]
. Therefore, either approach should decrease the risk for future atherosclerotic events by 40–50%[3]
. This fixed-dose strategy claims advantage because it derives directly from RCTs; however, it is often difficult to establish a firm baseline non-HDL-C level by which to judge percentage reduction and it is difficult to monitor the efficacy of therapy.
Benefits of lower cholesterol concentrations: ‘the lower, the better’
If epidemiological studies and RCTs are considered, a strong case can be made for ‘the lower, the better’ for cholesterol levels as individuals with the lowest cholesterol concentration have the lowest rates of ASCVD. This concept is supported by a recent meta-analysis of statin RCTs[25]
. A critical question of whether it is possible to attain greater risk reduction beyond that achieved by maximal statin therapy has been demonstrated in a study where enhanced risk reduction was achieved by adding non-statin drug ezetimibe to maximal statin therapy[26]
. Primary prevention trials with statins also support the concept of lower is better[25],
[27],
[28],
[29],
[30]
and, therefore, there is an opportunity to reintroduce cholesterol goals for lipid management. Advantages of atherogenic cholesterol goals are that they facilitate monitoring of patients for adherence and effectiveness of therapy and they help to ensure adequate cholesterol lowering to maximize risk reduction.
Benefits of longer cholesterol reduction: ‘the earlier the better’
Epidemiological studies[10]
, particularly genetic epidemiology studies[31],
[32]
, indicate that the longer serum cholesterol are kept at a low level, the greater will be the reduction in risk; a relationship particularly evident in populations with genetic mutations that reduce atherogenic cholesterol concentrations. In these studies[31]
, for every 1mmol/L lower the levels of cholesterol are at an early age, long-term risk is reduced two or three times more than that achieved in statin RCTs.
Cholesterol goals in primary prevention
Risk thresholds for statin therapy depend on a method called global risk assessment, which is based on epidemiologic study that defines the relationship between risk factors and cardiovascular events. Examples include: Framingham risk scoring[16]
, the ACC/AHA risk algorithm[21]
and QRISK[23]
. Both QRISK and ACC/AHA algorithms give similar but not identical results, but as QRISK has the advantage of being derived from a very large population database, it is the preferred method. Both ten-year-risk algorithms can be accessed online[33],
[34]
.
As mentioned before, there are three lines of evidence for identifying goals of cholesterol lowering in primary prevention. These include: (a) ‘the more (lowering), the better’ for relative risk reduction, (b) ‘the lower, the better’ for absolute risk reduction, and (c) ‘the earlier, the better’ for lifetime risk reduction. From these general axioms, several reasonable goals for each can be suggested. Table 3 lists four levels of risk for ASCVD: high ten-year risk, moderately high ten-year risk, lower ten-year risk but higher lifetime risk, and severe hypercholesterolaemia. At each level of risk, all non-lipid risk factors should also be treated to goal, and maximal lifestyle intervention should also be employed.
| Level of risk for ASCVD | Goals of therapy | Intensity of cholesterol-lowering therapy |
|---|---|---|
*Higher risk conditions include heavy smoking, diabetes, persistent hypertension, and persistently high non-HDL-C (>4.9 mmol/L) ** Atorvastatin 80 mg per day (or equivalent, see Table 2) ***Atorvastatin 10–20 mg per day (or equivalent, see Table 2) | ||
| All risk levels | Treat all risk factors to goals | |
| All risk levels | Maximal lifestyle intervention | |
High 10-year risk (>20%) | 45–60% non-HDL-C lowering or non-HDL-C <2.6 mmol/L | High-intensity** |
Moderately high 10-year risk (10–19%) | 35–60% non-HDL-C lowering or non-HDL-C <3.4 mmol/L | Moderate-intensity or high-intensity*** |
Lower 10-year risk (<10%) + high lifetime risk (35–50%) or + higher risk conditions* | Non-HDL-C goal <3.4 mmol/L | Moderate-intensity |
Severe hypercholesterolaemia (Non-HDL-C >190 mg/dL) | Non-HDL-C goal <3.4 mmol/L | High-intensity |
High-risk patients
Patients with established ASCVD typically have a ten-year risk for recurrent cardiovascular events >20%. For this reason, individuals without ASCVD whose ten-year risk is >20% can be said to be at high risk. At this level of risk, non-HDL-C should be reduced by 45–60%, or alternatively, to a non-HDL goal of <2.6 mmol/L. Since patients in this category have a risk comparable to those with established ASCVD, it is reasonable to reduce non-HDL-C to very low levels [2],
[19],
[25],
[30]
. To achieve this degree of non-HDL-C lowering, a high-intensity statin (e.g. atorvastatin 80mg or its equivalent, see table 2) is typically required.
Moderately high-risk patients
This category can be defined as a 10-year risk for ASCVD events of 10–19%, as calculated by a risk-assessment algorithm. At this risk level, non-HDL-C should be lowered by 35–60%, or alternatively, to a non-HDL-C goal of < 3.4 mmol/L. To achieve this level for non-HDL-C, a moderate-intensity statin (e.g. atorvastatin 20mg) is usually adequate.
Patients with lower ten-year risk but high lifetime risk
Those at lower ten-year risk (<10%) but at higher lifetime risk are potential candidates for statin therapy. A high lifetime risk can be defined as a risk for major ASCVD events of 35–50%. Lifetime risk can be estimated with either a QRISK algorithm[35]
or an ACC/AHA tool[34]
, or alternatively, any of several major risk conditions that confer a relatively high lifetime risk for ASCVD such as diabetes, metabolic syndrome, chronic kidney disease, persistent hypercholesterolaemia (LDL-C >4.1mmol/L or non-HDL-C >4.9mmol/L), heavy smoking, and/or poorly-controlled hypertension. Treating patients according to the presence or absence of these higher risk conditions may be more reliable than estimating risk by algorithm. Lower risk patients who have a high lifetime risk should be treated to a non-HDL-C goal of <3.4mmol/L and a moderate-intensity statin is usually sufficient.
Patients with severe hypercholesterolaemia
These patients can be defined as those with a non-HDL-C of >5.7mmol/L and are at high lifetime risk, even in the absence of other risk factors. For patients in this category, the aim should be to reduce non-HDL-C by 45–60%, or to attain a non-HDL-C level of <3.4mmol/L. A high-intensity statin is often required to achieve this goal, and add-on, non-statin drugs may be required as well.
Doctor-patient discussion
Statin therapy should not be initiated without a thorough discussion of the advantages and disadvantages with the patient[20],
[36]
. Since treatment with statins is a lifetime commitment, patients should be fully informed of the reasons for therapy, including efficacy, safety and cost considerations. Many patients are reluctant to use medication for long periods and a significant percentage will discontinue therapy[37]
. There are no simple rules to ensure prolonged adherence but the discussion should include a plan for regular monitoring and prescription renewal. The following describes several considerations for patient discussion:
Safety of statins
Patients should be informed that statins are usually safe for long-term use. There is no evidence that they have delayed serious side effects, such as cancer[38]
, but many patients still blame a variety of symptoms on their statins, even when the drug is not responsible, and they should be urged not to discontinue therapy without a full discussion with their healthcare professional. A more objective evaluation may reassure patients when statins are found not to be the cause of their symptoms. About 10–20% of patients complain of statin-associated muscle symptoms, which usually involve myalgia and/or muscle weakness[22],
[39],
[40]
. Statins very rarely cause severe myopathy (rhabdomyolysis), and some of the risk factors are listed in box 2. The majority of people with myalgia have no predisposing causes. Beyond myalgia, occasionally patients complain of cognitive dysfunction, which is an idiosyncratic response that may require discontinuation of the statin. Up to 10% of patients can develop some degree of hyperglycaemia and crossing the threshold for categorical diabetes is more likely to occur in patients with prediabetes[41]
. In patients with established diabetes, statins rarely worsen hyperglycaemia, and they clearly reduce the risk of ASCVD events. Peripheral neuropathy has been reported with statin therapy but it is very rare[42]
. Statins do not cause chronic liver disease or chronic kidney disease[38],
[43]
and, although they can cause a transaminase leak from the liver, this does not signify hepatotoxicity.
Box 2. Risk factors for statin-associated myopathy
- Advanced age (especially over 80 years) in patients (women more than men)
- Small body frame and frailty
- Multisystem disease (e.g. chronic renal insufficiency, especially caused by diabetes)
- Multiple medication
- Perioperative periods
- Specific concomitant medication or consumption as listed below (check specific statin package insert for warnings)
- Fibrates (especially gemfibrozil)
- Nicotinic acid (rarely)
- Ciclosporin
- Azole antifungals
- Itraconazole and ketoconazole
- Macrolide antibiotics
- Erythromycin and clarithromycin
- HIV protease inhibitors
- Nefazodone (antidepressant)
- Verapamil
- Amiodarone
- Large quantities of grapefruit juice (usually more than 1 litre per day)
- Alcohol abuse (independently predisposes to myopathy)
Alternatives to statin or add-on drugs
Statins are first-line cholesterol-lowering therapy but other ways to lower cholesterol levels deserve consideration. Lifestyle modifications (listed in box 3) are of prime concern and can lower atherogenic cholesterol by 10–15%[13]
. Bile acid resins are another consideration as they reduce LDL-C by 15–25% and reduce risk for ASCVD[13]
. Ezetimibe also lowers LDL-C by 15–25% and has been demonstrated to enhance ASCVD reduction when given with a high-dose statin[16]
. It is also a particularly attractive add-on drug to use with a moderate-intensity statin as this combination will produce the same cholesterol-lowering effect as a high-intensity statin. Fibrates and niacin are less effective LDL-lowering drugs but, when given alone, have been shown to reduce risk[13]
, although there is little evidence that they are effective add-on drugs to statin therapy to achieve further risk reduction[44]
. Non-statin drugs in primary prevention are mainly used for patients with statin intolerance. When given in combination, these drugs can achieve cholesterol-lowering similar to moderate-intensity statins.
Box 3. Recommended lifestyle modifications to reduce cholesterol levels
- Dietary cholesterol (<300 mg/day)
- Saturated fatty acids (<7% of total calories)
- Trans fatty acids (<1% of total calories)
- Dietary soluble fiber (10g/day)
- Dietary plant sterols (2g/day [optional])
- Total calorie intake (sustain desirable bodyweight)
- Regular physical activity (30 minutes/day)
Promising drugs to use in statin intolerant patients are PCSK9 inhibitors, which are monoclonal antibodies that remove a circulating protein called PCSK9[45]
. PCSK9 promotes degradation of LDL receptors and diminishes receptor-mediated reduction of LDL-C levels. Removal of PCSK9 from the circulation elevates LDL receptors and lowers LDL-C/non-HDL-C concentrations. Preliminary studies indicate that these drugs are highly effective for cholesterol lowering[46],
[47]
; however, while PCSK9 inhibitors have been approved by regulatory agencies, they are extremely expensive. Therefore, their immediate use will likely be limited to patients who have severe hypercholesterolaemia or who are at very high risk and cannot be adequately controlled with statins. If the price declines, they could be used routinely for patients with statin intolerance[48]
.
Cost effectiveness
If generic statins are available, cost is rarely an issue. Nonetheless, there are hidden costs to drug therapies, especially statins. Examples of hidden costs include regular monitoring for response, clinical management of side effects, and travel expenses to health care providers. In borderline risk patients, these factors may speak against initiation of therapy.
Older patients
Whether or not to use statins in older patients has been much discussed. Older individuals may be at a higher risk for statin-associated side effects, although the literature is limited. According to current risk algorithms that include advancing age as a major risk factor, older people will fall into higher risk categories and are therefore candidates for statin therapy. However, it is important to keep in mind that estimations of absolute risk by these algorithms are less reliable in the elderly than in middle-aged adults[49]
and, if they are used, many older people with little or no atherosclerosis will be treated unnecessarily. There is no doubt, however, that older people at high risk will benefit from statin therapy[50],[51]
. In RCTs, statins are efficacious for reducing ASCVD events, but are less effective for prolonging life, although of course, in older individuals in whom statins prevent ASCVD events, they may be life-saving. As always in geriatric medicines, various comorbidities can modify decisions about the initiation of statins[52]
.
Statins in women
For secondary prevention, statin use in women is well accepted and its benefit is well-documented[53]
. Use of statins in women for primary prevention has been a matter of some dispute[54]
. There seems little doubt that statins reduce risk for ASCVD in women at high risk, but the question is whether, for most women, risk is high enough to justify their use. Since women in general have lower absolute risk than men of comparable age, clinical judgement is required for deciding whether and when to initiate statins in women. Factors that favour their use include diabetes, heavy smoking, multiple risk factors (e.g. metabolic syndrome), hypercholesterolaemia and, perhaps, a strong family history of premature ASCVD.
Number needed to treat (NNT)
One parameter commonly used by physicians in discussion with patients is the number needed to treat (NNT), which is the number of people needed to treat to prevent one ASCVD event over a given period of time. The time frame can be five years (the average duration of clinical trials with cholesterol-lowering drugs), ten years (corresponding to usual absolute risk estimates), or over a lifetime. The purpose of the NNT is to give perspective. Table 4 shows the NNT for statins therapy at different risk levels over a ten-year period for moderate-intensity and high-intensity statins. Of course, the NNT over a lifetime will be much lower, depending on the age of onset of cholesterol-lowering therapy. A low lifetime NNT in patients with major risk factors provides the strongest rationale for a lifetime of statins therapy in primary prevention.
| Ten-year risk for ASCVD | NNT for moderate-intensity statins* | NNT for high-intensity statins** |
|---|---|---|
| * Assumes that moderate-intensity statins reduce risk by 33% | ||
| **Assumes that high-intensity statins reduce risk by 50% | ||
| 7.5% | 40 | 26 |
| 10% | 33 | 20 |
| 15% | 20 | 13 |
| 20% | 15 | 10 |
| 25% | 12 | 8 |
Subclinical atherosclerosis
If a risk algorithm is used for drug initiation, a sizeable number of individuals will be treated unnecessarily because they will have little or no atherosclerosis. If this population could be excluded from statin therapy, it would reduce the number of people for whom treatment is required for benefit. One way to avoid unnecessary drug treatment is to measure subclinical atherosclerosis and the best available method appears to be coronary artery calcium (CAC). Follow-up studies show that ten-year risk in individuals without any CAC is virtually zero[55]
. Those who have CAC scores in the range of 1–100 Agatston units have a relatively low ten-year risk, and risk rises substantially with increasing CAC scores. Table 5 illustrates the potential utility of subclinical atherosclerosis for selection of patients for statin therapy.
| CAC Score (Agatston units) | Ten-year risk for CHD (45–54 years) | Ten-year risk for CHD (>75 years) |
|---|---|---|
| 0 | 1% | 1% |
| 1–99 | 4% | 7% |
| > 100 | 17% | 18% |
Emerging risk factors
Some investigators favour using emerging risk factors when making decisions about the use of cholesterol-lowering drugs in primary prevention. For example, elevations of C-reactive protein and/or fibrinogen are useful for identifying patients with metabolic syndrome, particularly when they occur in the presence of abdominal obesity and prediabetes. If a strong family history of premature ASCVD is present it can count as a high-risk condition and an elevation of lipoprotein(a) would suggest a need for more intensive LDL-lowering therapy. Measurements of any, or all, of these emerging risk factors are options in risk assessment; however, they are not routinely recommended by guideline committees because of their uncertain relation to ASCVD.
Conclusion
Statins are indicated for most patients with established ASCVD but their indications for primary prevention are much more nuanced. Most authorities agree that statin therapy is justified for individuals who are at high risk, or moderately high risk, of ASCVD. Risk status can be estimated by standard algorithms or based on the presence, or absence, of major risk factors. Unfortunately, standard algorithms are less robust in older people whose estimated risk is relatively high but whose actual risk may be low. Many older people have little atherosclerosis and will not benefit from statin treatment; this is particularly the case in older women. The only solution to this conundrum is to actually measure the severity of subclinical atherosclerosis but, at present, such measurements are not widely available in general practice. Therefore, physicians must exercise their best clinical judgement and discuss the advantages and disadvantages of statin therapy with their patients. For middle-aged people with major risk factors, estimates of lifetime risk may be more useful than ten-year risk estimates for deciding whether and when to introduce statin therapy. On account of of growing evidence for overall safety and falling costs, the use of statins in primary prevention is increasingly feasible. In the future, new RCTs may better define the scope of primary prevention with cholesterol-lowering drugs, particularly statins.
Scott M Grundy: Center for Human Nutrition and Department of Internal Medicine, UT Southwestern Medical Center, 5323 Harry Hines Blvd., Suite Y3.206, Dallas, TX 75390-9052, United States. Correspondence to:
scott.grundy@utsouthwestern.edu
Financial and conflicts of interest disclosure
The author has no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilised in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Endo A. The discovery and development of HMG-CoA reductase inhibitors. J Lipid Res 1992;33(11):1569–1582. Available at: http://www.jlr.org/content/33/11/1569.long (accessed February 2016).
[2] Smith SC Jr, Benjamin EJ, Bonow RO et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol 2011;58(23):2432–2446. doi: 10.1016/j.jacc.2011.10.824. Erratum in: J Am Coll Cardiol 2015;65(14):1495.
[3] Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5.
[4] Wald NJ & Law MR. A strategy to reduce cardiovascular disease by more than 80%. The BMJ 2003;326(7404):1419. doi: 10.1136/bmj.326.7404.1419 Erratum in: The BMJ. 2003;327(7415):586. The BMJ. 2006;60(9):823.
[5] Newman DH, Saini V, Brody H et al. Statins for people at low risk of cardiovascular disease. The Lancet 2012;380(9856):1814; author reply 1817–1818. doi: 10.1016/S0140-6736(12)62020-0.
[6] McGill HC Jr, McMahan CA & Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation 2008;117(9):1216–1227. doi: 10.1161/CIRCULATIONAHA.107.717033.
[7] Stary HC, Chandler AB, Glagov S et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb 1994;14(5):840–856. doi: 10.1161/01.atv.14.5.840
[8] Stary HC, Chandler AB, Dinsmore RE et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995;15(9):1512–1531. doi: 10.1161/01.atv.15.9.1512
[9] Libby P. Collagenases and cracks in the plaque. J Clin Invest 2013;123(8):3201–3203. doi: 10.1172/JCI67526
[10] Law MR, Wald NJ & Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease? The BMJ 1994;308(6925):367–372. doi: 10.1136/bmj.308.6925.367
[11] Stamler J, Daviglus ML, Garside DB et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000;284(3):311–318. doi: 10.1001/jama.284.3.311
[12] Grundy SM. Age as a risk factor: you are as old as your arteries. Am J Cardiol 1999;83(10):1455–1457. doi: 10.1016/S0002-9149(99)00125-3
[13] National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. Available at:http://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/Circulation-2002-ATP-III-Final-Report-PDF-3143.pdf (accessed February 2016).
[14] Zannad F, De Backer G, Graham I et al.; ESC Working Group on CardioVascular Pharmacology and Drug Therapy. Risk stratification in cardiovascular disease primary prevention — scoring systems, novel markers, and imaging techniques. Fundam Clin Pharmacol 2012;26(2):163–174. doi: 10.1111/j.1472-8206.2011.01023.x.
[15] McPherson R, Pertsemlidis A, Kavaslar N et al. A common allele on chromosome 9 associated with coronary heart disease. Science 2007;316(5830):1488–1491. doi: 10.1126/science.1142447
[16] Wilson PW, D’Agostino RB, Levy D et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837
[17] Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS), Catapano AL, Reiner Z, De Backer G et al.; ESC Committee for Practice Guidelines 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis 2011;217:S1–44. doi: 10.1016/j.atherosclerosis.2011.06.012
[18] Conroy RM, Pyörälä K, Fitzgerald AP et al.; SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3
[19] Anderson TJ, Grégoire J, Hegele RA et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29(2):151–167. doi: 10.1016/j.cjca.2012.11.032
[20] Stone NJ, Robinson JG, Lichtenstein AH et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002
[21] Goff DC Jr, Lloyd-Jones DM, Bennett G et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005
[22] National Institute for Health and Care Excellence (NICE). Cardiovascular disease: risk assessment and reduction, including lipid modification. NICE Guidelines [CG181]. July 2014. Available at: www.nice.org.uk/guidance/cg181 (accessed January 2016).
[23] Hippisley-Cox J, Coupland C, Robson J et al. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. The BMJ 2010;341:c6624. doi: 10.1136/bmj.c6624
[24] Taylor F, Huffman MD, Macedo AF et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5
[25] Boekholdt SM, Hovingh GK, Mora S et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: a meta-analysis of statin trials. J Am Coll Cardiol 2014;64(5):485–494. doi: 10.1016/j.jacc.2014.02.615
[26] Cannon CP, Blazing MA, Giugliano RP et al.; IMPROVE-IT Investigators. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med 2015;372(25):2387–2397. doi:10.1056/NEJMoa1410489
[27] Shepherd J, Cobbe SM, Ford I et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001
[28] Downs JR, Clearfield M, Weis S et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615
[29] Sever PS, Dahlöf B, Poulter NR et al.; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149–1158. doi: 10.1016/s0140-6736(03)12948-0
[30] Ridker PM, Danielson E, Fonseca FA et al.; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646
[31] Cohen JC, Boerwinkle E, Mosley TH Jr et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354(12):1264–1272. doi: 10.1056/nejmoa054013
[32] Brown MS & Goldstein JL. Biomedicine: Lowering LDL–not only how low, but how long? Science 2006;311(5768):1721–1723. doi: 10.1126/science.1125884
[33] ClinRisk. QRISK® 2015 risk calculator. Available at: www.qrisk.org (accessed January 2016).
[34] American College of Cardiology and American Heart Association. ASCVD Risk Estimator. Available at: //tools.acc.org/ASCVD-Risk-Estimator/ (accessed February 2016).
[35] ClinRisk. QRISK® - lifetime cardiovascular risk calculator. Available at: www.qrisk.org/lifetime/ (accessed February 2016).
[36] Reiner Ž. Statins in the primary prevention of cardiovascular disease. Nat Rev Cardiol 2013;10(8):453–464. doi: 10.1038/nrcardio.2013.80
[37] Degli Esposti L, Saragoni S, Batacchi P et al. Adherence to statin treatment and health outcomes in an Italian cohort of newly treated patients: results from an administrative database analysis. Clin Ther 2012;34(1):190–199. doi: 10.1016/j.clinthera.2011.12.011
[38] Å imić I & Reiner Ž. Adverse effects of statins — myths and reality. Curr Pharm Des. 2015;21(9):1220–1226. doi: 10.2174/1381612820666141013134447
[39] Stroes ES, Thompson PD, Corsini A et al.; European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012–1022. doi: 10.1093/eurheartj/ehv043
[40] Harris LJ, Thapa R, Brown M et al. Clinical and laboratory phenotype of patients experiencing statin intolerance attributable to myalgia. J Clin Lipidol 2011;5(4):299–307. doi: 10.1016/j.jacl.2011.05.005
[41] Betteridge DJ & Carmena R. The diabetogenic action of statins — mechanisms and clinical implications. Nat Rev Endocrinol 2016;12:99–110. doi: 10.1038/nrendo.2015.194
[42] Chong PH, Boskovich A, Stevkovic N et al. Statin-associated peripheral neuropathy: review of the literature. Pharmacotherapy 2004;24(9):1194–1203. doi: 10.1592/phco.24.13.1194.38084
[43] Smith SC Jr & Grundy SM. Reply: Statin dose based on limited evidence. J Am Coll Cardiol 2015;65(7):760–761. doi: 10.1016/j.jacc.2014.11.042
[44] HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, Hopewell JC et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955
[45] Horton JD, Cohen JC & Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res 2009;50:S172–177. doi: 10.1194/jlr.R800091-JLR200
[46] Robinson JG, Farnier M, Krempf M et al.; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015 16;372(16):1489–1499. doi: 10.1056/NEJMoa1501031
[47] Reiner Ž. PCSK9 inhibitors–past, present and future. Expert Opin Drug Metab Toxicol 2015;11(10):1517–1521. doi: 10.1517/17425255.2015.1075506
[48] Lipinski MJ, Benedetto U, Escarcega RO et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2015:ehv563. doi: 10.1093/eurheartj/ehv563
[49] Navar-Boggan AM, Peterson ED, D’Agostino RB Sr et al. Using age and sex-specific risk thresholds to guide statin therapy: one size may not fit all. J Am Coll Cardiol 2015;65(16):1633–1639. doi: 10.1016/j.jacc.2015.02.025
[50] Teng M, Lin L, Zhao YJ et al. Statins for Primary Prevention of Cardiovascular Disease in Elderly Patients: Systematic Review and Meta-Analysis. Drugs Aging 2015;32(8):649–661. doi: 10.1007/s40266-015-0290-9
[51] Savarese G, Gotto AM Jr, Paolillo S et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62(22):2090–2099. doi:10.1016/j.jacc.2013.07.069
[52] Reiner Z. Primary prevention of cardiovascular disease with statins in the elderly. Curr Atheroscler Rep 2014;16(7):420. doi: 10.1007/s11883-014-0420-6
[53] Cholesterol Treatment Trialists’ (CTT) Collaboration, Fulcher J, O’Connell R, Voysey M et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. The Lancet 2015;385(9976):1397–1405. doi: 10.1016/S0140-6736(14)61368-4
[54] Do Statins have a Role in Primary Prevention? Therapeutics Letter #48. June 30 2003. Available at: http://www.ti.ubc.ca/2003/06/30/do-statins-have-a-role-in-primary-prevention/ (accessed January 2016).
[55] Tota-Maharaj R, Blaha MJ, Blankstein R et al. Association of coronary artery calcium and coronary heart disease events in young and elderly participants in the multi-ethnic study of atherosclerosis: a secondary analysis of a prospective, population-based cohort. Mayo Clin Proc 2014;89(10):1350–1359. doi: 10.1016/j.mayocp.2014.05.017