Key points
- Oral administration is currently not possible for biologics.
- Barriers in the gastrointestinal tract severely limit the systemic absorption of biologics.
- Different research strategies have been explored to enable oral delivery of biologics.
- Recent advances in materials and increased knowledge of the physiological barriers have produced advances and significant technologies with potential to reach the clinic.
Introduction
Biologics — medicines that include products from living organisms, such as recombinant proteins, peptides and vaccines — have revolutionised the management of a range of a range of conditions, such as diabetes, inflammatory diseases (e.g. rheumatoid arthritis and inflammatory bowel disease [IBD]) and cancer. Although they have been in clinical use for a long time — almost 100 years in the case of insulin — their development and use have increased dramatically over the past two decades, owing to advances in biotechnology and a new understanding of biology and disease processes. In 2018, eight out of ten top selling medicines (global sales in US$) were biologics[1]
.
Biologics differ from chemically derived ‘conventional’ medicines in many ways, with implications on production, administration, clinical efficacy and cost. Compared with small-molecule drugs, such as aspirin, biotherapeutics are generally of significant higher molecular weight and have an inherently heterogeneous structure. As large and complex molecules, biologics are extremely sensitive to the physical and chemical conditions of the gastrointestinal (GI) environment. It is because of this sensitivity that, with a few exceptions, biologics are currently administered by injection.
However, oral administration is the most convenient and preferred method of drug administration[2],[3],[4],[5]
. Ingestion also offers additional benefits compared with administration by injection: for example, oral administration of insulin more closely mimics the physiology of endogenous insulin secreted by the pancreas, which leads to decreased levels of systemic insulin, and minimises hypoglycaemic episodes and weight gain problems[6]
. In addition, oral administration of insulin also reduces needle-related complications and cost.
Despite research into the oral delivery of biologics taking place for almost a century, the current clinical reality remains unchanged in terms of therapeutic administration[7],[8],[9]
. However, the proliferation of biologics available on the market has intensified this research activity. Coupled with recent advances in materials, research into oral delivery of biologics is increasingly producing more clinically relevant drug-delivery technologies, with the potential to make oral administration of biologics a viable option.
This article provides an overview of the drug delivery of biologics and recent advances in this field. The process of producing biologics is not within the scope of this article.
Physiological barriers to oral delivery of biologics
A major challenge in achieving clinically relevant oral delivery of biologics is overcoming the multiple physiological barriers in the GI tract (GIT), which are designed to prevent the uptake of foreign materials, including harmful pathogens or their products, from the external environment (i.e. gut lumen; see Figure 1).
A major chemical barrier is the pH-induced proteolysis of proteins into constituent amino acids, dipeptides and tripeptides[10]
. The biochemical barriers include proteolytic enzymes in the gut lumen (e.g. pepsin, trypsin, chymotrypsin), proteolytic enzymes at the brush border membrane (i.e. endopeptidases) and efflux pump P-glycoprotein[10]
.
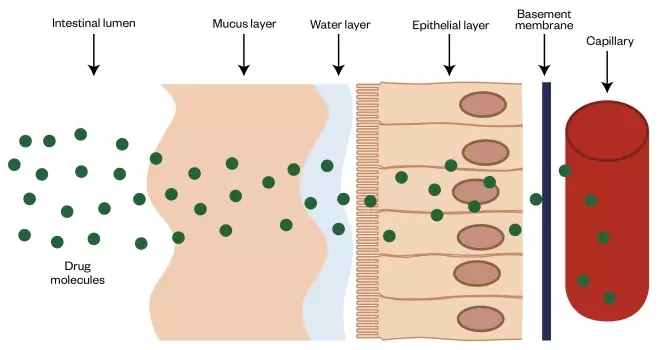
Figure 1: Physiological barriers to the absorption of biologics in the intestine
Following oral administration, systemic absorption of biologics is limited by several physiological barriers, including stomach acid and enzymes (not shown). Furthermore, mucus hinders the diffusion of macromolecules. The intestinal epithelium is not usually penetrable by hydrophilic macromolecules. The extracellular matrix-based basement membrane and capillary endothelium may present additional barriers to intestinal absorption of biologics.
However, the largest and most important barrier for absorption of biologics is the intestinal epithelium. Although it is a single cell thick, its cells are arranged to form a near-continuous cell membrane barrier facing the lumen. Furthermore, the mucus layer of varying thickness (depending on the region of the gut) that sits above the epithelium may also act as a barrier, hindering the diffusion of biologics to the underlying epithelium[10]
. Basement membranes, which are located between the epithelia and connective tissue as thin and specialised sheets of extracellular matrix, can hamper the penetration of macromolecules into the space beneath the epithelium, thus limiting systemic absorption[11],[12]
. These factors significantly contribute to biopharmaceuticals having oral bioavailability of less than 1%[13]
.
Strategies for improving oral delivery of biologics
Protect the biologic from acid and enzymatic degradation
One strategy that can enhance the bioavailability of biologic medicines is reducing acid degradation. This can be achieved by delivery within enteric-coated systems, which are clinically well-established and, therefore, will not be discussed in this article[14]
.
Co-administration of protein and peptide drugs with protease inhibitors can protect biotherapeutics from the proteolytic enzymes present in the intestinal environment[15]
. It is also possible to modify the chemical structures of some biologics, particularly peptides, in order to improve their stability in the GI fluids (e.g. this could be achieved via the ‘cyclisation’ approach[15]
).
Some biologics have higher intrinsic physicochemical stability against enzymatic degradation in the GIT and may show potential for oral delivery. Examples include shark- and llama-derived antibody fragments, with the latter being investigated as oral delivery anti-tumour necrosis factor-alpha biologics for the treatment of IBD[16]
.
It must be noted that protection of the biologic drug from acid and enzymatic degradation is an important requirement and any strategies that seek to improve oral delivery of biologics, as discussed below, must ensure that this requirement is also met.
Increase the contact time of the biologic with the absorptive epithelium
The aim of this strategy is to prevent the luminal loss of the medicine, which is important considering the length of the intestines, and present it at high concentrations in close proximity to the absorptive epithelium.
‘Mucoadhesive’ materials are typically polymers capable of interacting with mucus via ionic and non-ionic interactions; these can help prolong the medicine’s residence time at the absorption site, leading to enhanced absorption[17]
. Natural mucoadhesive polymers include chitosan, pectin, gelatine, sodium alginate, guar gum and xanthan gum[18]
, while synthetic mucoadhesive polymers include cellulose derivates, poly(acrylic acid) polymers, poly(ethylene glycol), poly(ethylene oxide), poly(vinyl pyrrolidone) and poly(vinyl alcohol)[19]
. Many of these materials have been investigated for oral delivery of biologics with varied success[20]
.
A mucoadhesive ‘transdermal patch-like’ system has the ability to improve oral delivery of the therapeutic polypeptide, salmon calcitonin (sCT)[21]
, based on the delivery of mucoadhesive polymers — carbopol 934, pectin and sodium carboxymethylcellulose — via in gastro-resistant hard gelatin capsules. This system has demonstrated significant enhancement of intestinal sCT absorption in vivo. Gupta et al. have investigated similar mucoadhesive patches for oral delivery of exenatide and insulin[22]
. Surgical placement of these systems in the rat jejunum resulted in a 42% decrease in blood glucose, while the insulin solution-treated group (control) showed no such effect. The relative bioavailability of insulin and exenatide increased dramatically compared with intestinal injections (13-fold and 80-fold, respectively)[22]
.
While mucoadhesive systems have demonstrated potential for enabling oral delivery of biologics in vitro and in vivo, potential challenges facing this strategy include limited efficacy, particularly with larger biologics (e.g. monoclonal antibodies). Simply prolonging the residence time of the biotherapeutic at the absorptive surface may not be sufficient to achieve clinically relevant improvement of bioavailability. This is understandable given the limited ability of hydrophilic drugs with molecular weight orders of magnitude above 500 Daltons to traverse the intestinal epithelium. It is also currently unclear how the intestinal mucus turnover can affect the action of these systems[20]
. Furthermore, there may be potential issues with application of such systems in diseases associated with mucus defects (e.g. IBD).
Make the mucosal barrier more permeable
These are the most commonly researched strategies for improving the oral bioavailability of biologics, as both the intestinal mucus barrier and the epithelial barrier can be modified.
Modifying the mucus barrier by using mucolytic (mucus-breaking) agents, such as N-acetylcysteine, can improve the diffusion of large molecule biologics. However, since it is the epithelium that typically is the rate-limiting barrier, not the mucus, it is usually more advantageous to manipulate this. The epithelial barrier can be modified via several chemical absorption enhancers, including surfactants and other materials that open epithelial tight junctions[23]
.
Surfactants
These contain both a hydrophilic and hydrophobic component, and can adsorb onto interfaces of a system and alter the interfacial free energy and tension. This results in fluidisation of intestinal epithelial plasma membrane, but also transient opening of epithelial tight junctions, thereby facilitating permeation of macromolecules[24]
.
Surfactants based on medium-chain fatty acids (e.g. sodium caprate, sodium caprylate and N-[8-(2-hydroxybenxoyl) amino] caprylate [SNAC]), bile salts and acylcarnitines are the main candidates currently being used in the development of oral peptide formulations[25]
. Technologies that utilise these materials that are currently undergoing clinical trials include the ‘gastro-intestinal permeating technology’ (Novo Nordisk) and the ‘eligen’ technology (Novo Nordisk). A SNAC formulation for the oral delivery of the long-acting GLP-1 analogue, semaglutide (Novo Nordisk), for type 2 diabetes mellitus was recently reported to have successfully completed the first phase IIIa trial. The 703-person study achieved its primary objective, demonstrating improvements in HbA1c level for three orally-administered doses of semaglutide (3mg, 7mg and 14mg) compared with placebo. Moreover, vitamin B12 tablets containing large doses of SNAC are already on the market[26]
.
Mycapssa (Chiasma) capsules are currently being investigated in three global phase III studies with promising potential. Chiasma, a biopharmaceutical company based in Israel, developed the ‘transient permeability enhancer’ (TPE) technology currently used in Mycapssa capsule formulations for the maintenance therapy of adult patients with acromegaly[27]
. The peptide, octreotide — a somatostatin analogue — is the active drug in this formulation. TPE technology can enhance the oral bioavailability of octreotide owing to the combination of pharmaceutical excipients. This composition creates an oily suspension of hydrophilic particles in a hydrophobic matrix[28]
. Octreotide, along with sodium caprylate and other excipients, are solubilised in the hydrophilic component. The surfactants contained in this formulation trigger the temporary expansion of tight junctions and allow the drug, which is protected from the digestive enzymes, to permeate the intestinal epithelial membrane and reach the bloodstream[28]
.
Tight junction-opening permeation enhancers
Many materials capable of opening epithelial tight junctions, including surfactants, have been identified over decades of research in this area. Epithelial tight junction-opening is a potentially useful approach to increase the permeability of the intestinal epithelium as the medicine can avoid entering the epithelial cells and be present in an enzyme-rich cytoplasmic environment during its absorption process.
The process involves widening the space between adjacent epithelial cells (the paracellular space), which is normally too small to accommodate biologics. However, tight junction-opening must be reversible so that the physiological role of the epithelium is maintained as a tight barrier. Although many materials have shown capability to reversibly open epithelial tight junctions, chitosans are probably the most extensively researched compounds[29]
. These are derived from the natural polymer, chitin, which is found in cell walls of fungi and the exoskeletons of arthropods, such as crustaceans and insects. Various forms of chitosans have been investigated; however, as with other permeation enhancers, the long-term effects of repeatedly opening intestinal epithelial tight junctions are unknown and require further study[29]
.
It must be noted that drug delivery approaches that employ chemicals to modify the mucosal barrier — namely absorption enhancers, such as surfactants and tight junction-opening agents — rely on concentration-dependent effects on barrier permeability. Therefore, clinical implications of this method may relate to potential variability in absorption as a result of fasted and fed state, as well as the volume of water used for swallowing solid dosage forms. In addition, the long-term effects of repeated alteration of GIT permeability currently remain unclear and require careful evaluation.
Make the biologic drug or drug delivery system more permeable
Depending on the nature of the biologic, it is possible via chemical modification to alter the molecule to impart its epithelial-permeating properties[15]
. It is also possible to increase the ability of the biotherapeutic to cross the intestinal epithelium by attaching it to another molecule that is capable of doing so. Typically, this ‘transport-enabling’ molecule traverses the intestinal epithelium by a specific receptor expressed in the intestinal epithelial cells. The two entities can be attached through chemical attachment (conjugation) or via biotechnology-mediated fusion technologies. Examples of transport-enabling molecules include other peptides or proteins that utilise biological transport processes to traffic across the epithelium[30]
.
In addition to altering the biologic to improve its likelihood to cross the intestinal barrier, researchers have incorporated biotherapeutics into drug carrier systems that can traverse the intestinal barrier[31]
. Biologic carriers for this purpose are typically of nanometre scale and based on biodegradable polymeric nanoparticles, which have numerous advantages. For example, some nanoparticles offer protection of the therapeutic drug from acid and enzymes present in the GIT. Moreover, selective drug delivery can be achieved through targeting of specific receptors located on the surface of intestinal epithelial cells. However, similar to large molecule biologics, nanoparticle carriers are typically poorly absorbed across the intestinal mucosa[32]
. Nanoparticles may diffuse poorly in the intestinal mucus and are not capable of crossing the intestinal epithelium. For this reason, nanoparticle-based drug carriers for the oral delivery of biologics are engineered with specific materials on their surface that act as ligands for biological transport receptors expressed in intestinal epithelial cells. Such delivery systems, including nanoparticles exploiting the intestinal epithelial transport pathways of immunoglobulin G (IgG) and vitamin B12, have been explored by several research groups[23]
.
One biological transport pathway that has shown considerable potential for intestinal nanoparticle transport is the neonatal Fc receptor (FcRn), which is present in the human intestinal epithelium and participates in the intestinal transport of IgG and serum albumin[33]
. FcRn-targeted polymer nanoparticles showed potential for oral insulin delivery[31]
. In mice, these orally administered nanoparticles crossed the intestinal epithelium and reached the systemic circulation with higher absorption efficiency than nanoparticles not targeted to the FcRn system. The nanoparticles containing insulin induced a prolonged hypoglycaemic effect in mice expressing the receptor, compared with the control group (FcRn-knockout mice)[31]
. In another study, FcRn-targeted polymer nanoparticles were studied for oral delivery of exenatide[34]
. These systems moved across the intestinal epithelium faster and to a greater extent when compared with unmodified nanoparticles. Delivery of exenatide by these nanoparticles resulted in an extended hypoglycaemia compared with subcutaneous exenatide injection[34]
.
Despite the potential advantages and promising results at the preclinical research stage, development of nanomedicine-based strategies faces several challenges:
- Nanoparticle-based carriers may suffer from low therapeutic loading capacity — particularly with larger biologics, such as monoclonal antibodies[23]
; - The delivery capacity may also be an issue, with biological pathways exploited by these systems typically having a low transport capacity[35]
; - The complex nanocarriers may undergo extensive degradation or alteration in the GIT, in the presence of highly complex intestinal biofluid. The main issue is the attachment or adsorption of materials as normal constituents of intestinal biofluid, including peptides and proteins, adsorbing on the surface of nanocarriers, which influences their ability to target biological receptors and utilise these systems for epithelial transport[23]
.
Overcome the mucosal barrier using ‘smart’ ingestible devices
In addition to being capable of protecting the therapeutic from the hostile environment of the GIT, ingestible ‘smart’ devices enhance the intestinal absorption of biologics through different means, including ultrasound and microneedles[25]
. The microneedle oral delivery technology is being developed in the United States by Rani Therapeutics and, according to the company, preclinical research so far has shown encouraging results[36]
.
The technology involves a capsule designed to remain intact in the stomach; once in the small intestine, it injects the medicine into the intestinal wall (see Figure 2). This is a painless process owing to the absence of pain receptors in the intestinal mucosa and has shown impressive insulin bioavailability that is equivalent to or better than subcutaneous injections. The advantage of this technology is that as well as the delivery of low-to-medium molecular weight biologics, it may also be able to deliver larger biologics, such as antibodies[36]
.
The capsules are initially coated by a pH-responsive coating to aid ingestion. When the pill has reached the desired location in the GIT, the coating dissolves, releasing the microneedles. In the case of systems with hollow microneedles, the drug reservoir is compressed through peristalsis, releasing the drug through the needles. For systems with solid microneedles, the drug is formulated into the microneedles that penetrate the tissue and break off from the pill, leaving the needle to release the drug in a controlled manner based on the needle formulation[37]
. After drug release, the microneedles remain lodged in the GI tissue until biodegradation.
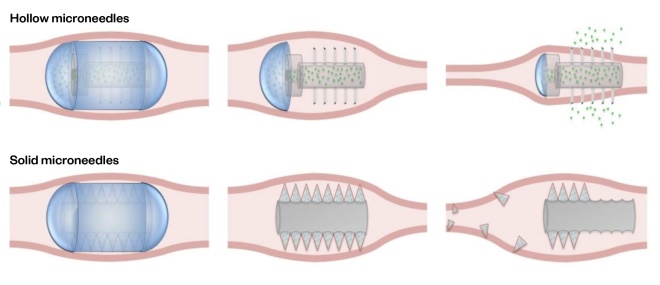
Figure 2: Therapeutic use concept of hollow and solid microneedle pills in the gastrointestinal tract
Source: Reproduced with permission from J Pharm Sci
[37]
In hollow microneedles, the drug reservoir is compressed through peristalsis, releasing the drug through the needles. In solid microneedles, the drug is formulated into the microneedles. These penetrate the tissue and break off from the pill, leaving the needle to release the drug in a controlled manner.
A recent landmark study reported a drug delivery system that resembles the leopard tortoise (Stigmochelys pardalis) — a tortoise with a steeply domed shell that it uses to self-orient should it roll onto its back. Termed the ‘self-orienting millimetre-scale applicator’ (SOMA), this novel system for the oral delivery of biologics uses this same shape and low centre of gravity to self-orientate in a correct position and physically insert a biodegradable microneedle through the stomach mucosa for systemic administration of biotherapeutics[38]
(see Figure 3). When the SOMA was loaded with human insulin and administered to swine, a blood-glucose lowering effect was observed, indicating successful drug delivery though the mucosal layer[38]
.
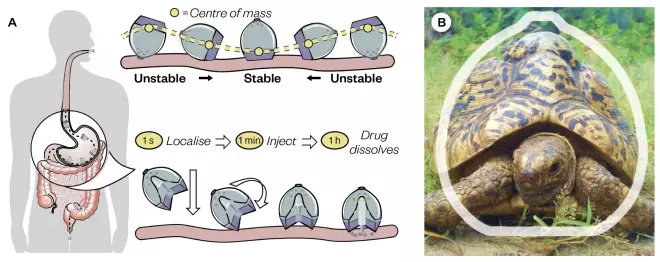
Figure 3: Mechanical active pharmaceutical ingredient localisation and injection for oral gastric delivery
Source: Adapted from Science
[38]
. Image: M.M.Karim / Wikimedia Commons, CC-BY-SA 2.5
(A) The self-orienting millimetre-scale applicator (SOMA) localises to the stomach lining, orients its injection mechanism toward the tissue wall and injects a drug payload through the mucosa. The drug dissolves and the rest of the device passes out of the body. (B) A comparison between the shape of the leopard tortoise (Stigmochelys pardalis) and that of the SOMA. The SOMA quickly orients and remains stable in the stomach environment after reaching its preferred orientation.
Potential for clinical translation of oral biologics delivery strategies
Devices for oral delivery of biologics are showing significant potential, but research in this area is still in its infancy. While many of the drug delivery strategies discussed above have shown positive results and potential in vivo and in vitro, they are yet to be used in patients. Unfortunately, with many of the delivery approaches discussed above, safety and efficacy are often mutually exclusive and, therefore, such strategies are unlikely to progress to the clinic. Furthermore, it is well known that small intestinal epithelial damage is caused by many of the permeation enhancers in current oral peptide clinical trials[17]
. Although tissue damage is often temporary and reparable, it remains unknown if chronic repeat dosing of such absorption enhancers could overcome the body’s repair mechanisms.
A safer alternative could be one that relies on improving the intestinal absorption of biologics by exploiting biological transport processes to achieve delivery without damaging the tissue; however, these are likely to be faced by limited capacity and may be best suited for more potent biologics. Such devices need to clearly demonstrate safety on repeated administration in humans; it seems that efficacy is not an issue. Furthermore, the costs of these technologies are currently unclear, but are likely to be high in the short-to-medium term — in which case, it will be critical to give careful consideration to the selection of the biologic, disease area and patient population for use of these drug delivery systems.
Conclusion
Research into oral delivery of biologics has made considerable progress, yet has not produced a significant impact in the clinic to date. The lack of clinical translation success in this area, which partly reflects the highly effective physiological barriers in the GIT, has usually been related to the safety of drug delivery approaches. However, increased knowledge of the physiological barriers, coupled with unprecedented recent developments in materials, are propelling advances in this area, and are likely to make oral delivery of biologics a clinical reality.
Financial and conflicts of interest disclosure
The authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript. No writing assistance was used in the production of this manuscript.
References
[1] Urquhart L. Top drugs and companies by sales in 2018. Nat Rev Drug Discov 2019;18(245). doi: 10.1038/d41573-019-00049-0
[2] Eek D, Krohe M, Mazar I et al. Patient-reported preferences for oral versus intravenous administration for the treatment of cancer: a review of the literature. Patient Prefer Adherence 2016;10:1609–1621. doi: 10.2147/ppa.s106629
[3] Pridgen EM, Alexis F & Farokhzad OC. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Expert Opin Drug Deliv 2015;12(9):1459–1473. doi: 10.1517/17425247.2015.1018175
[4] Sosnik A & Augustine R. Challenges in oral drug delivery of antiretrovirals and the innovative strategies to overcome them. Adv Drug Deliv Rev 2016;103:105–120. doi: 10.1016/j.addr.2015.12.022
[5] GarcÃa-Pérez LE, Alvarez M, Dilla T et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther 2013;4(2):175–194. doi: 10.1007/s13300-013-0034-y
[6] Gedawy A, Martinez J, Al-Salami H & Dass CR. Oral insulin delivery: existing barriers and current counter-strategies. J Pharm Pharmacol 2018;70(2):197–213. doi: 10.1111/jphp.12852
[7] Harrison GA. Insulin in alcoholic solution by the mouth. BMJ 1923;2(3286):1204–1205. doi: 10.1136/bmj.2.3286.1204
[8] Murlin JR, Sutter CC, Allen RS & Piper HA. Some favorable effects from the alimentary administration of insulin. Endocrinology 1924;8(3):331–339. doi: 10.1210/endo-8-3-331
[9] Salen E. Einige Versuche mit peroraler Darreichung von Insulin [Attempts with oral insulin administration]. Acta Medica Scandinavica 1924;60(1):74–87. doi: 10.1111/j.0954-6820.1924.tb15268.x
[10] Khanvilkar K, Donovan MD & Flanagan DR. Drug transfer through mucus. Adv Drug Deliv Rev 2001;48(2–3):173–193. doi: 10.1016/s0169-409x(01)00115-6
[11] Mantaj J, Abu-Shams T, Enlo-Scott Z et al. Role of the basement membrane as an intestinal barrier to absorption of macromolecules and nanoparticles. Mol Pharm 2018;15(12):5802–5808. doi: 10.1021/acs.molpharmaceut.8b01053
[12] Vllasaliu D, Falcone FH, Stolnik S & Garnett M. Basement membrane influences intestinal epithelial cell growth and presents a barrier to the movement of macromolecules. Exp Cell Res 2014;323(1):218–231. doi: 10.1016/j.yexcr.2014.02.022
[13] Mahato RI, Narang AS, Thoma L & Miller DD. Emerging trends in oral delivery of peptide and protein drugs. Crit Rev Ther Drug Carrier Syst 2003;20(2–3):153–214. doi: 10.1615/critrevtherdrugcarriersyst.v20.i23.30
[14] Maher S, Ryan B, Duffy A & Brayden DJ. Formulation strategies to improve oral peptide delivery. Pharm Pat Anal 2014;3(3):313–336. doi: 10.4155/ppa.14.15
[15] Bruno BJ, Miller GD & Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv 2013;4(11):1443–1467. doi: 10.4155/tde.13.104
[16] Crowe JS, Roberts KJ, Carlton TM et al. Preclinical development of a novel, orally-administered anti-tumour necrosis factor domain antibody for the treatment of inflammatory bowel disease. Sci Rep 2018;8(1):4941. doi: 10.1038/s41598-018-23277-7
[17] Bernkop-Schnürch A. Mucoadhesive systems in oral drug delivery. Drug Discov Today Technol 2005;2(1):83–87. doi: 10.1016/j.ddtec.2005.05.001
[18] Cook SL, Bull SP, Methven L et al. Mucoadhesion: a food perspective. Food Hydrocolloids 2017;72:281–296. doi: 10.1016/j.foodhyd.2017.05.043
[19] Rossi S, Vigani B, Sandri G et al. Recent advances in the mucus-interacting approach for vaginal drug delivery: from mucoadhesive to mucus-penetrating nanoparticles. Expert Opin Drug Deliv 2019;16(8):777–781. doi: 10.1080/17425247.2019.1645117
[20] Homayun B, Lin X & Choi HJ. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019;11(3):129. doi: 10.3390/pharmaceutics11030129
[21] Gupta V, Hwang BH, Lee J et al. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J Control Release 2013;172(3):753–762. doi: 10.1016/j.jconrel.2013.09.004
[22] Gupta V, Hwang BH, Doshi N et al. Delivery of exenatide and insulin using mucoadhesive intestinal devices. Ann Biomed Eng 2016;44(6):1993–2007. doi: 10.1007/s10439-016-1558-x
[23] Vllasaliu D, Thanou M, Stolnik S & Fowler R. Recent advances in oral delivery of biologics: nanomedicine and physical modes of delivery. Expert Opin Drug Deliv 2018;15(8):759–770. doi: 10.1080/17425247.2018.1504017
[24] Anderberg EK, Nystrom C & Artursson P. Epithelial transport of drugs in cell culture. VII: Effects of pharmaceutical surfactant excipients and bile acids on transepithelial permeability in monolayers of human intestinal epithelial (Caco-2) cells. J Pharm Sci 1992;81(9):879–887. doi: 10.1002/jps.2600810908
[25] McCartney F, Gleeson JP & Brayden DJ. Safety concerns over the use of intestinal permeation enhancers: a mini-review. Tissue Barriers 2016;4(2):e1176822. doi: 10.1080/21688370.2016.1176822
[26] Castelli MC, Wong DF, Friedman K & Riley MG. Pharmacokinetics of oral cyanocobalamin formulated with sodium N-[8-(2-hydroxybenzoyl)amino]caprylate (SNAC): an open-label, randomized, single-dose, parallel-group study in healthy male subjects. Clin Ther 2011;33(7):934–945. doi: 10.1016/j.clinthera.2011.05.088
[27] Melmed S, Popovic V, Bidlingmaier M et al. Safety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trial. J Clin Endocrinol Metab 2015;100(4):1699–1708. doi: 10.1210/jc.2014-4113
[28] Tuvia S, Pelled D, Marom K et al. A novel suspension formulation enhances intestinal absorption of macromolecules via transient and reversible transport mechanisms. Pharm Res 2014;31(8):2010–2021. doi: 10.1007/s11095-014-1303-9
[29] Thanou M, Verhoef JC & Junginger HE. Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev 2001;52(2):117–126. doi: 10.1016/s0169-409x(01)00231-9
[30] Amet N, Wang W & Shen WC. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release 2010;141(2):177–182. doi: 10.1016/j.jconrel.2009.09.007
[31] Pridgen EM, Alexis F, Kuo TT et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery. Sci Transl Med 2013;5(213):213ra167. doi: 10.1126/scitranslmed.3007049
[32] Cao SJ, Xu S, Wang HM et al. Nanoparticles: oral delivery for protein and peptide drugs. AAPS PharmSciTech 2019;20(5):190. doi: 10.1208/s12249-019-1325-z
[33] Kuo TT, Baker K, Yoshida M et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol 2010;30(6):777–789. doi: 10.1007/s10875-010-9468-4
[34] Shi Y, Sun X, Zhang L et al. Fc-modified exenatide-loaded nanoparticles for oral delivery to improve hypoglycemic effects in mice. Sci Rep 2018;8(1):726. doi: 10.1038/s41598-018-19170-y
[35] Shen S, Wu Y, Liu Y & Wu D. High drug-loading nanomedicines: progress, current status, and prospects. Int J Nanomedicine 2017;12:4085–4109. doi: 10.2147/ijn.s132780
[36] BioSpace. First human study of “robotic” RaniPill capsule to replace injections announced by Rani Therapeutics. 2019. Available at: https://www.biospace.com/article/releases/first-human-study-of-and-quot-robotic-and-quot-ranipill-capsule-to-replace-injections-announced-by-rani-therapeutics/ (accessed January 2020)
[37] Traverso G, Schoellhammer CM, Schroeder A et al. Microneedles for drug delivery via the gastrointestinal tract. J Pharm Sci 2015;104(2):362–367. doi: 10.1002/jps.24182
[38] Abramson A, Caffarel-Salvador E, Khang M et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 2019;363(6427):611–615. doi: 10.1126/science.aau2277