Latest:
- Results from the REMAP-CAP trial show that among critically ill patients with COVID-19 randomised to receive 1 or more therapeutic interventions, treatment with an IL-6 receptor antagonist had a greater than 99.9% probability of improved 180-day mortality compared with patients randomised to the control, and treatment with an antiplatelet had a 95.0% probability of improved 180-day mortality compared with patients randomised to the control (Writing Committee for the REMAP-CAP Investigators, 16 December 2022);
- Favipiravir does not improve clinical outcomes in all patients admitted to hospital with COVID-19, however, patients younger than 60 years might have a beneficial clinical response (Shah et al, 14 Dec 2022);
- New therapy added: N-acetylcysteine;
- NAC was beneficial in reducing the mortality rate in patients with COVID-19 and inflammatory parameters, and a reduction in the development of severe respiratory failure; however, it did not affect the length of hospital stay or the need for ICU admission (Panahi et al, 10 Dec 2022).
Researchers around the world are working at record speed to find the best ways to treat and prevent COVID-19, from investigating the possibility of repurposing existing drugs to searching for novel therapies against the virus.
Current approaches to COVID-19 therapies generally fall into two categories: antivirals — which prevent the virus from multiplying — and immune modulators — which help the immune system to fight the virus or stop it from overreacting dangerously. Some potential therapies act in a different way or via multiple mechanisms.
There are thousands of clinical trials of COVID-19 therapies taking place across the world. On 15 June 2020, the European Medicines Agency said it was in discussion with the developers of 132 potential COVID-19 treatments[1].
This article collates the main treatments being studied, the evidence supporting their use and the trials they are being evaluated in. It will be updated on a regular basis.
Only evidence from randomised controlled trials comprising more than 100 participants is included, with the exception of select observational studies that have had a significant influence on ongoing research.
Contents
- Remdesivir
- Chloroquine/hydroxychloroquine
- Amodiaquine
- Artesunate
- Lopinavir/ritonavir combination
- Favipiravir
- Ribavirin
- EIDD-2801
- Niclosamide
- Nitazoxanide
- Oseltamivir
- Ivermectin
- AT-527
- Molnupiravir
- Paxlovid
Immune modulators:
- Dexamethasone
- Hydrocortisone
- Convalescent plasma
- Budesonide (inhaled)
- AZD7442
- Azithromycin
- Doxycycline
- Interferons
- Tocilizumab
- Sarilumab
- Regdanvimab
- Canakinumab
- Anakinra
- Baricitinib
- Ruxolitinib
- Tofacitinib
- Acalabrutinib
- Imatinib
- Brensocatib
- Ravulizumab
- Namilumab
- Infliximab
- Adalimumab
- Otilimab
- Medi3506
- Monoclonal antibody cocktails
- Bamlanivimab (monotherapy)
- Etesevimab (monotherapy)
- Sotrovimab
- Leronlimab
- Risankizumab
- Lenzilumab
- IMU-838
- CD24Fc
Other or multiple mechanisms:
- Colchicine
- Dimethyl fumarate
- Angiotensin-converting-enzyme inhibitors/angiotensin II receptor blockers
- Statins
- Aspirin
- Clopidogrel
- Anticoagulants
- Bemcentinib
- Omeprazole
- Famotidine
- Zilucoplan
- Ascorbic acid/vitamin C
- Vitamin D3
- Aviptadil
- Tradipitant
- Nitric oxide
- Fluvoxamine
- Proxalutamide
- Ruconest
- TRV027
- Ciclesonide
- Sabizabulin
- N-acetylcysteine
Antivirals

Source: Shutterstock.com
Remdesivir
- Broad-spectrum antiviral originally developed to treat hepatitis C and was then tested against Ebola;
- In vitro activity against SAR-CoV-2;
- Some evidence of efficacy against COVID-19 in humans;
- Available on a ‘compassionate use’ basis in many countries;
- First COVID-19 treatment to be made available for use in the UK outside a clinical trial;
- In the EU, remdesivir is now licensed for the treatment of COVID-19 in adults and adolescents with pneumonia requiring supplemental oxygen;
- On 20 November 2020, the WHO issued a conditional recommendation against the use of remdesivir in hospitalised patients, regardless of disease severity, as there is currently no evidence that remdesivir improves survival and other outcomes in these patients.
Evidence
- Final results from the Solidarity trial suggest that remdesivir has no significant effect on patients with COVID-19 who are already being ventilated, and, among other hospitalised patients, it has a small effect against death or progression to ventilation (or both) (WHO Solidarity Trial Consortium, 2 May 2022);
- In an analysis of 562 non-hospitalised participants, who were at high risk for COVID-19 progression, randomly assigned in a 1:1 ratio to receive a three-day course of remdesivir or placebo, remdesivir demonstrated a statistically significant 87% reduction in risk for the composite primary endpoint of COVID-19 related hospitalisation or all-cause death by day 28 (Gottlieb et al, 22 December 2021);
- No clinical benefit was observed from the use of remdesivir in patients who were admitted to hospital for COVID-19, were symptomatic for more than 7 days, and required oxygen support (Ader et al, 14 September 2021)
- Neither remdesivir nor hydroxychloroquine affected viral clearance in hospitalized patients with COVID-19 (Barratt-Due et al, 13 July 2021);
- Roche announces that the global phase III randomised, double-blind, multicentre REMDACTA study of tocilizumab plus remdesivir, versus placebo plus remdesivir, did not meet its primary endpoint. This was measured by improved time to hospital discharge up to day 28 in patients with severe COVID-19 pneumonia receiving standard of care (11 March 2021);
- Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status among patients with COVID-19, notably among those receiving high-flow oxygen or noninvasive ventilation. The combination was associated with fewer serious adverse events (Kalil et al, 4 March 2021);
- Interim results from the Solidarity trial suggest that remdesivir has little or no effect on mortality in patients who are hospitalised with COVID-19 (WHO Solidarity Trial Consortium, 2 December 2020);
- Remdesivir was superior to placebo in shortening the time to recovery in adults hospitalised with COVID-19 and had evidence of lower respiratory tract infection (Beigel et al, 8 October 2020)
- In moderate COVID-19, a 10-day course of remdesivir did not have a statistically significant difference in clinical status compared with standard care at 11 days after initiation of treatment. Patients randomized to a 5-day course of remdesivir had a statistically significant difference in clinical status compared with standard care, but the difference was of uncertain clinical importance (Spinner et al, 21 August 2020)
- No significant efficacy difference between five and ten day courses of remdesivir in patients with severe COVID-19 (Goldman et al, 27 May 2020);
- “No statistically significant differences” for mortality and serious adverse events in COVID-19 patients treated with remdesivir (National Institute for health and Care Excellence [NICE]);
- First randomised trial of remdesivir suggests antiviral drug is not associated with significant clinical benefits, but numerical reduction in time to clinical improvement suggests more research needed (Wang et al, 29 April 2020).
Ongoing trials
- ACTIV-3: Therapeutics for Inpatients With COVID-19 (TICO);
- Trial of Treatments for COVID-19 in Hospitalized Adults (DISCOVERY);
- SOLIDARITY clinical trial for COVID-19 treatments.
Chloroquine/hydroxychloroquine
- Antimalarials with in vitro activity against various viruses, including SAR-CoV-2 — the virus that causes COVID-19;
- Anecdotal evidence in humans;
- The US Food and Drug Administration has cautioned against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems;
- In June 2020, all UK clinical trials using hydroxychloroquine to treat or prevent COVID-19 were instructed by the Medicines and Healthcare products Regulatory Agency (MHRA) to stop recruiting further participants;
- Approved for the treatment of rheumatoid arthritis and lupus.
Evidence
- Neither remdesivir nor hydroxychloroquine affected viral clearance in hospitalized patients with COVID-19 (Barratt-Due et al, 13 July 2021);
- Neither hydroxychloroquine nor lopinavir-ritonavir showed any significant benefit for decreasing COVID-19–associated hospitalisation or other secondary clinical outcomes, a randomised controlled trial has concluded (Reis et al, 22 April 2021);
- WHO guideline development panel make a strong recommendation against the use of hydroxychloroquine for individuals who do not have COVID-19 (2 March 2021);
- Rigorous randomised controlled trial among persons with recent exposure excluded a clinically meaningful effect of hydroxychloroquine as postexposure prophylaxis to prevent SARS-CoV-2 infection (Barnabas et al, 8 December 2020);
- Interim results from the Solidarity trial suggest that remdesivir has little or no effect on mortality in patients who are hospitalised with COVID-19 (WHO Solidarity Trial Consortium, 2 December 2020);
- Post-exposure therapy with hydroxychloroquine did not prevent SARS-CoV-2 infection or symptomatic COVID-19 in healthy persons exposed to a PCR-positive case patient (Mitjà et al. 24 November 2020);
- Among adults hospitalised with respiratory illness from COVID-19, treatment with hydroxychloroquine, compared with placebo, did not significantly improve clinical status at day 14 (Self et al, 9 November 2020);
- Pre-exposure prophylaxis with hydroxychloroquine once or twice weekly did not significantly reduce laboratory-confirmed COVID-19 or COVID-19-compatible illness among healthcare workers (Rajasingham et al, 17 October 2020);
- Among patients hospitalised with COVID-19, those who received hydroxychloroquine did not have a lower incidence of death at 28 days than those who received usual care (The RECOVERY collaborative group, 8 October 2020);
- Among hospital-based health care workers, daily hydroxychloroquine did not prevent SARS-CoV-2 infection, although the trial was terminated early and may have been underpowered to detect a clinically important difference (Abella et al, 30 September 2020)
- Among patients hospitalised with mild-to-moderate COVID-19, the use of hydroxychloroquine, alone or with azithromycin, did not improve clinical status at 15 days as compared with standard care (Cavalcanti et al, 23 July 2020)
- Hydroxychloroquine did not substantially reduce symptom severity in outpatients with early, mild COVID-19 (Skipper et al, 16 July 2020)
- UK Medicines and Healthcare products Regulatory Agency (MHRA) suspends recruitment to COVID-19 hydroxychloroquine trials (5 June 2020)
- Preliminary results from ‘Randomised Evaluation of COVID-19 Therapy’ (RECOVERY) trial (5 June 2020): no significant difference in mortality rate at 28 days (25.7% hydroxychloroquine vs. 23.5% usual care; hazard ratio 1.11 [95% confidence interval 0.98–1.26]; P =0.10);
- Hydroxychloroquine did not prevent illness compatible with COVID-19 or confirmed infection when used as postexposure prophylaxis within four days after exposure (Boulware et al, 3 June 2020);
- No evidence of benefit of hydroxychloroquine or chloroquine, when used alone or with a macrolide. Each of these drug regimens was associated with decreased in-hospital survival and an increased frequency of ventricular arrhythmias (Mehra et al, 22 May 2020). Study retracted by The Lancet on 5 June 2020 following concerns about the data;
- Not enough data available to support the routine use of hydroxychloroquine and chloroquine as therapies for COVID-19 (Chowdhury et al, 29 May 2020);
- Administration of hydroxychloroquine did not result in a significantly higher probability of negative conversion than standard of care alone (Tang et al, 14 May 2020).
Ongoing trials
- Chemoprophylaxis for COVID-19 infectious disease (the PROLIFIC trial) – suspended;
- An adaptive phase 2/3, randomised, open-label study assessing efficacy and safety of hydroxychloroquine for hospitalised patients with moderate to severe COVID-19;
- Health Care Worker Prophylaxis Against COVID-19 (HERO).
Amodiaquine
- An antimalarial similar in structure and activity to chloroquine; effective against some chloroquine-resistant strains;
- Found to be highly effective at preventing viral entry; these results have been validated in cultured cells and in a small animal model of COVID-19 using infectious SARS-CoV-2 virus.
Ongoing trials
Artesunate
- A water soluble derivative of artemisinin used in the treatment of severe malaria;
- Artemisinin-based combination therapies have demonstrated in vitro inhibition of SARS-CoV-2 as well as anti-inflammatory effects.
Ongoing trials
Lopinavir/ritonavir combination
- HIV type 1 aspartate protease inhibitors, indicated for treatment of HIV infection in combination with other antiretroviral drugs;
- Lopinavir has in vitro inhibitory activity against SARS-CoV, the virus that causes severe acute respiratory syndrome (SARS);
- Ritonavir is combined with lopinavir to increase its half-life;
- Recommended for use in COVID-19 in several countries, including Italy and France.
Evidence
- Neither hydroxychloroquine nor lopinavir-ritonavir showed any significant benefit for decreasing COVID-19–associated hospitalisation or other secondary clinical outcomes, a randomised controlled trial has concluded (Reis et al, 22 April 2021);
- Interim results from the Solidarity trial suggest that remdesivir has little or no effect on mortality in patients who are hospitalised with COVID-19 (WHO Solidarity Trial Consortium, 2 December 2020);
- In patients admitted to hospital with COVID-19, lopinavir–ritonavir was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death (The RECOVERY collaborative group, 5 October 2020).
- Following a review of emerging data from the RECOVERY trial, researchers concluded that there was no beneficial effect of lopinavir/ritonavir on 28-day mortality in patients hospitalised with COVID-19 compared to usual care alone (Horby et al, 29 June 2020);
- Evidence that early treatment with triple antiviral therapy of interferon (IFN) beta-1b, lopinavir/ritonavir, and ribavirin — alongside standard care — is safe and shortens duration of viral shedding compared with lopinavir-ritonavir alone (average 7 days vs. 12 days), in patients with mild-to-moderate COVID-19 (Hung et al, 8 May 2020);
- Some evidence that lopinavir/ritonavir initiation within 12 days after symptom onset is associated with shorter time to clinical improvement. No significant differences in reduction of viral RNA load, duration of viral RNA detectability, duration of oxygen therapy, duration of hospitalisation, or time from randomization to death. Lopinavir/ritonavir stopped early in 13 patients because of adverse effects (Cao et al, 7 May 2020).
Favipiravir
- Broad-spectrum antiviral with in vitro activity against various viruses, including coronaviruses;
- Licensed in Japan and China for treatment of influenza;
- Not currently included in any of the UK trials for COVID-19.
Evidence
- Favipiravir does not improve clinical outcomes in all patients admitted to hospital with COVID-19, however, patients younger than 60 years might have a beneficial clinical response (Shah et al, 14 Dec 2022);
- Phase III PRESECO (PREventing SEvere COVID-19) clinical trial evaluating oral antiviral, favipiravir, for the treatment of mild-to-moderate COVID-19 did not achieve statistical significance on the primary endpoint of time to sustained clinical recovery (12 Nov 2021).
Ongoing trials
- PRINCIPLE (added April 2021);
- Phase III Clinical Study to Evaluate the Performance and Safety of Favipiravir in COVID-19;
- A Randomized Controlled Trial for Favipiravir Tablets Combine with Chloroquine Phosphate in the Treatment of Novel Coronavirus Pneumonia (COVID-19);
- Favipiravir Combined with Tocilizumab in the Treatment of novel coronavirus pneumonia (COVID-19) — a multicentre, randomised, controlled trial;
- The Efficacy and Safety of Favipiravir for novel coronavirus–infected pneumonia — a multicentre, randomised, open, positive, parallel-controlled clinical study.
Ribavirin
- Broad-spectrum antiviral used to treat hepatitis C, respiratory syncytial virus (RSV) and bronchiolitis;
- In vitro activity against SARS-CoV, the virus that causes severe acute respiratory syndrome (SARS);
- Some evidence of efficacy as an adjunct therapy in SARS;
- Evidence from mouse models in SARS-CoV suggested it could increase infectivity.
Evidence
- Evidence that early treatment with triple antiviral therapy of IFN beta-1b, lopinavir-ritonavir, and ribavirin — alongside standard care — is safe and shortens duration of viral shedding compared with lopinavir-ritonavir alone (average 7 days vs. 12 days), in patients with mild to moderate COVID-19 (Hung et al, 8 May 2020).
EIDD-2801
- Investigational oral nucleoside analogue with broad-spectrum antiviral activity against RNA viruses, including influenza and coronaviruses like SARS and Middle East respiratory syndrome (MERS).
Ongoing trials
Niclosamide
- Anti-helminthic drug with potential antiviral activity against SARS-CoV-2;
- Unlicensed in the UK.
Ongoing trials
- Prophylaxis for vulnerable patients at risk of COVID-19 infection (PROTECT-V) trial;
- Niclosamide for mild-to-moderate COVID-19.
Nitazoxanide
- A broad-spectrum antiparasitic and antiviral medication used for the treatment of various helminthic, protozoal, and viral infections;
- Has yielded successful results in vitro against previous coronaviruses.
Evidence
- In patients with mild COVID-19, symptom resolution did not differ between nitazoxanide and placebo groups after 5 days of therapy. However, early nitazoxanide therapy was safe and reduced viral load significantly (Rocco et al, 24 Dec 2020).
Ongoing trials
- ANTICOV.
Oseltamivir
- A neuraminidase inhibitor approved for the treatment of influenza A and B;
- Several clinical trials are evaluating the effectiveness of oseltamivir in treating SARS-CoV-2 both alone and in combination with other drugs.
Ongoing trials
- IMU-838 and Oseltamivir in the Treatment of COVID-19 (IONIC);
- Hydroxychloroquine, Oseltamivir and Azithromycin for the Treatment of COVID-19 Infection: An RCT (PROTECT);
- Favipiravir, Protease Inhibitors, Oseltamivir -Gpo, Hydroxychloroquine for Treatment of COVID-19 (FIGHT-COVID-19);
- A Prospective/Retrospective,Randomized Controlled Clinical Study of Antiviral Therapy in the 2019-nCoV Pneumonia.
Ivermectin
- Anti-parasitic agent shown to have antiviral activity against a broad range of viruses;
- Unlicensed in the UK;
- Shown to inhibit the SARS-CoV-2 virus in vitro;
- On 22 March 2021, the European Medicines Agency advises against use of ivermectin for the prevention or treatment of COVID-19 outside randomised clinical trials;
- At the beginning of August 2021, a preprint from Elgazzar et al. regarding the promising potential of ivermectin on COVID-19 patients, was retracted due to suspicion of data manipulation.
Evidence
- In a randomized trial of metformin, ivermectin, and fluvoxamine none prevented the occurrence of hypoxemia, an emergency department visit, hospitalisation, or death associated with COVID-19 (Bramante et al, 18 August 2022);
- Treatment with ivermectin did not result in a lower incidence of medical admission to a hospital due to progression of COVID-19 or of prolonged emergency department observation among outpatients with an early diagnosis of COVID-19 (Reis et al, 5 May 2022);
- In an open-label randomised clinical trial of high-risk patients with COVID-19 in Malaysia, a 5-day course of oral ivermectin administered during the first week of illness did not reduce the risk of developing severe disease compared with standard of care alone (Chee Loon Lim, et al, 18 February 2022);
- A randomised, double-blind, placebo-controlled study found that ivermectin had no significant effect on preventing hospitalisation of patients with COVID-19. Patients who received ivermectin required invasive mechanical ventilatory support earlier in their treatment. No significant differences were observed in any of the other secondary outcomes (Vallejos et al, 2 July 2021);
- Patients with mild-to-moderate COVID-19 infection treated with ivermectin plus doxycycline recovered earlier, were less likely to progress to more serious disease, and were more likely to be COVID-19 negative by RT-PCR on day 14 (Mahmud et al, 13 May 2021);
- Findings from a randomised controlled trial of 476 patients do not support the use of ivermectin for treatment of mild COVID-19, although larger trials may be needed to understand effects on other clinically relevant outcomes (López-Medina et al, 4 March 2021);
- Ivermectin is suggested to be a promising, effective and safe chemoprophylactic drug in the management of COVID-19 (Shoumann et al, 1 Feb 2021).
Ongoing trials
- PRINCIPLE (23 June 2021);
- ANTICOV;
- Ivermectin in Adults With Severe COVID-19;
- Ivermectin Treatment Efficacy in COVID-19 High Risk Patients (I-TECH);
- Prophylaxis for COVID-19: Ivermectin in Close Contacts of COVID-19 Cases (IVERNEX-TUC);
- An Outpatient Clinical Trial Using Ivermectin and Doxycycline in COVID-19 Positive Patients at High Risk to Prevent COVID-19 Related Hospitalization;
- Ivermectin as a Novel Therapy in COVID-19 Treatment.
AT-527
- An orally administered, direct-acting antiviral currently in development;
- Targets SARS-CoV-2 ribonucleic acid (RNA) polymerase (nsp12), a highly conserved gene responsible for both viral RNA replication and transcription;
- It is anticipated that ATR-527’s antiviral activity will be effective against the emerging strains of the virus.
Ongoing trials
- Study to Evaluate the Effects of AT-527 in Non-Hospitalized Adult Patients With Mild or Moderate COVID-19;
- Safety and Efficacy of AT-527 in Subjects With Moderate Coronavirus Disease (COVID-19);
- MORNINGSKY trial.
Molnupiravir
- Investigational, orally administered form of a potent ribonucleoside analog that inhibits the replication of SARS-CoV-2;
- Shown to be active in several preclinical models of SARS-CoV-2, including for prophylaxis, treatment, and prevention of transmission;
- Pre-clinical and clinical data have shown molnupiravir to be active against the most common SARS-CoV-2 variants;
- On 4 November 2021, molnupiravir became the first oral antiviral to be approved by the Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of COVID-19;
- On 19 November 2021, the European Medicines Agency human medicines committee issued advice on the use of molnupiravir for the treatment of COVID-19. The medicine, which is currently not authorised in the EU, can be used to treat adults with COVID-19 who do not require supplemental oxygen and who are at increased risk of developing severe COVID-19, it should be administered as soon as possible after diagnosis of COVID-19 and within 5 days of the start of symptoms. The medicine, which is available as capsules, should be taken twice a day for 5 days.
Evidence
- Published results of the MOVe-OUT study of molnupiravir including data from all enrolled participants (n=1,433) show that molnupiravir reduced the risk of hospitalisation or death from 9.7% in the placebo group (68/699) to 6.8% (48/709) in the molnupiravir group, for an absolute risk reduction of 3.0% (95% confidence interval [CI]: 0.1, 5.9; nominal p-value=0.0218) and a relative risk reduction of 30% (relative risk 0.70; 95% CI: 0.49, 0.99) (Bernal et al, 16 December 2021);
- A planned interim analysis of a global, phase III, randomised controlled trial showed that molnupiravir reduced the risk of hospitalisation or death by approximately 50%; 7.3% of patients who received molnupiravir were either hospitalised or had died by day 29 compared with 14.1% of placebo-treated patients.
Ongoing trials
- ‘Platform adaptive trial of novel antivirals for early treatment of COVID-19 In the community’ (PANORAMIC);
- Efficacy and Safety of Molnupiravir (MK-4482) in Non-Hospitalized Adult Participants With COVID-19 (MK-4482-002);
- Study of MK-4482 for Prevention of Coronavirus Disease 2019 (COVID-19) in Adults (MK-4482-013) (MOVe-AHEAD).
Paxlovid (nirmatrelvir +ritonavir)
- Investigational oral antiviral designed to block the activity of a key enzyme needed for SARS-CoV-2 to multiply;
- Administered along with a low dose of ritonavir, an older medication widely used in combination treatments for HIV infection;
- On 19 November 2021, the European Medicines Agency (EMA) said it was reviewing currently available data on the use of Paxlovid (PF-07321332/ritonavir);
- On 16 December 2021, the EMA’s human medicines committee issued advice saying the treatment could be used to treat adults with COVID-19 who do not require supplemental oxygen and who are at increased risk of progressing to severe disease. Paxlovid should be administered as soon as possible after diagnosis of COVID-19 and within 5 days of the start of symptoms;
- On 31 December 2021, Paxlovid was approved by the MHRA for the early treatment of COVID-19;
- On 27 January 2022, UK guidance recommended Paxlovid as as a first-line treatment to patients with hospital-onset COVID-19, as well as to eligible COVID-19 patients in the community.
Evidence
- Treatment of symptomatic COVID-19 with nirmatrelvir plus ritonavir resulted in a risk of progression to severe Covid-19 that was 89% lower than the risk with placebo, without evident safety concerns (Hammond et al, 16 February 2022);
- Interim analysis result showed that there was an 89% reduction in the risk of COVID-19-related hospitalisation or death from any cause in patients who received PF-07321322/ritonavir within three days of developing symptoms, compared with those who received placebo. Overall, 0.8% (3/389) of patients who received the drugs were hospitalised by day 28 compared with 7% (27/385) of patients who received the placebo (5 November 2021).
Ongoing trials
- A Study of PF-07321332/Ritonavir in Nonhospitalized High Risk Adult Participants With COVID-19;
- A Study of PF-07321332/Ritonavir in Non-hospitalized Low-Risk Adult Participants With COVID-19;
- A Post-Exposure Prophylaxis Study of PF-07321332/Ritonavir in Adult Household Contacts of an Individual With Symptomatic COVID-19.
Immune modulators

Shutterstock.com
Dexamethasone
- Steroid that reduces inflammation by mimicking anti-inflammatory hormones produced by the body;
- Indicated for the suppression of inflammatory and allergic disorders;
- Only suitable for people who are already in hospital and receiving oxygen or mechanical ventilation;
- It is the first drug to be shown to improve survival in COVID-19;
- Approved for NHS use by UK government.
Evidence
- Among patients with COVID-19 and severe hypoxemia, 12 mg/d of dexamethasone compared with 6 mg/d of dexamethasone did not result in statistically significantly more days alive without life support at 28 days. However, the trial may have been underpowered to identify a significant difference (The COVID STEROID 2 Trial Group, 21 October 2021);
- In patients hospitalised with COVID-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support (RECOVERY Collaborative Group, 25 February 2021).
- Intravenous dexamethasone plus standard care, compared with standard of care alone, resulted in a statistically significant increase in the number of days alive and free of mechanical ventilation over 28 days. (Tomazini et al, 2 September 2020);
- Preliminary results from the RECOVERY trial suggest that dexamethasone reduced deaths by 35% in ventilated patients and by 20% in other patients receiving oxygen only. There was no benefit among those patients who did not require respiratory support (Horby et al, 17 July 2020).
Ongoing trials
- Dexamethasone for COVID-19;
- Effect of Two Different Doses of Dexamethasone in Patients With ARDS and COVID-19 (REMED);
- Low or High Dose of Dexamethasone in Patients With Respiratory Failure by COVID-19 (HIGHLOWDEXA).
Hydrocortisone
- Steroid that reduces inflammation by mimicking anti-inflammatory hormones produced by the body;
- Used for a variety of conditions including adrenocortical insufficiency, rheumatoid arthritis, dermatitis, asthma and chronic obstructive pulmonary disorder;
- Commonly used to manage septic shock in patients with COVID-19;
- Evidence regarding corticosteroid use for severe COVID-19 is limited.
Evidence
- Patients with severe COVID-19 who are treated intravenously with the steroid, hydrocorticosone, are up to 93% more likely to have a better outcome compared to patients who are not given the drug, principal findings from the REMAP-CAP trial suggest. However, the trial was stopped early and no treatment strategy met pre-specified criteria for statistical superiority (Angus et al, 2 September 2020);
- Low-dose hydrocortisone did not significantly reduce treatment failure in patients with COVID-19–related acute respiratory failure; however, the study was stopped early and was therefore likely underpowered (Dequin et al, 2 September 2020).
Ongoing trials
- Hydrocortisone for COVID-19 and Severe Hypoxia (COVID STEROID);
- REMAP-CAP;
- ACTIV-3b: Therapeutics for Severely Ill Inpatients With COVID-19 (TESICO);
- RECOVERY.
Budesonide (inhaled)
- Inhaled budesonide is often used to treat asthma and chronic obstructive pulmonary disease, with no serious side-effects associated with short-term use;
- In some patients with COVID-19, the body’s immune response to the virus can cause high levels of inflammation that can damage cells in the airways and lungs. Inhaling budesonide into the airways targets anti-inflammatory treatment where it is needed most, and can potentially minimise any lung damage that might otherwise be caused by the virus;
- In updated guidance, published in December 2021, the National Institute for Health and Care Excellence said there was “no statistically significant difference” between patients treated with inhaled budesonide for COVID-19 and those who received usual care.
Evidence
- In updated guidance, published on 14 December 2021, NICE said there was “no statistically significant difference” in the likelihood of hospitalisation, need for mechanical ventilation, admission to intensive care or death, between patients with COVID-19 who used inhaled budesonide alongside usual care, compared with those who had usual care alone;
- The European Medicines Agency’s COVID-19 taskforce (COVID-ETF) has advised healthcare professionals that there is currently ‘insufficient evidence’ that inhaled corticosteroids, such as budesonide, are beneficial for people with COVID-19 (27 May 2021);
- Interim findings from the PRINCIPLE trial suggest that budesonide shortens recovery time in non-hospitalised patients with COVID-19 by a median of three days (12 April 2021);
- Phase II randomised controlled trial suggests that early administration of inhaled budesonide reduces the likelihood of needing urgent medical care and reduces time to recovery after early COVID-19 (Ramakrishnan et al, 9 April 2021).
Ongoing trials
- PRINCIPLE;
- Trial Evaluating the Efficacy of Local Budesonide Therapy in the Management of Hyposmia in COVID-19 Patients Without Signs of Severity (COVIDORL);
- Inhaled Corticosteroid Treatment of COVID-19 Patients With Pneumonia;
- Protective Role of Inhaled Steroids for COVID-19 Infection (INHASCO)
Convalescent plasma
- Antibody-rich plasma of someone who has recovered from COVID-19;
- There is some evidence suggesting possible benefits of convalescent plasma in patients with COVID-19, but available data to date are largely from case reports or series; confirmation from additional randomised controlled studies is required (Malani et al, 12 June 2020);
- Has been approved for use in critically ill patients in the United States and UK.
Evidence
- In participants with COVID-19, most of whom were unvaccinated, the administration of convalescent plasma within 9 days after the onset of symptoms reduced the risk of disease progression leading to hospitalisation (Sullivan et al, 30 March 2022);
- A meta-analysis of eight randomised controlled trials found no association of COVID-19 convalescent plasma with better clinical outcomes for the typical patient (Troxel et al, 25 January 2022);
- A randomised controlled trial found that convalescent plasma did not meet prespecified outcomes for efficacy, but high-titer convalescent plasma may have benefited hospitalised patients with COVID-19 early in the pandemic when other treatments were not in use, suggesting a heterogenous treatment effect over time (Ortigoza et al, 13 December 2021);
- A meta-analysis of 33 randomised controlled trials concluded that convalescent plasma treatment of patients with COVID-19 did not reduce all-cause mortality. The authors said that the results provided strong evidence that convalescent plasma treatment for patients with COVID-19 should not be used outside of randomised trials (Axfors et al, 20 November 2021);
- Among critically ill adults with confirmed COVID-19, treatment with 2 units of high-titer, ABO-compatible convalescent plasma had a low likelihood of providing improvement in the number of organ support–free days (Writing Committee for the REMAP-CAP Investigators, 4 October 2021);
- Published results from the RECOVERY trial show that, among patients hospitalised with COVID-19, high-titre convalescent plasma did not improve survival or other prespecified clinical outcomes (RECOVERY Collaborative Group, 14 May 2021);
- A randomised double-blind controlled trial in adults with severe COVID-19 suggested that use of convalescent plasma was not associated with significant improvement in clinical status at day 28. However, a significant improvement in mortality was observed, which, the authors said, warranted further evaluation (O’Donnell et al. 11 May 2021);
- Early administration of high-titer convalescent plasma against SARS-CoV-2 to mildly ill infected older adults reduced the progression of COVID-19 (Libster et al, 18 February 2021);
- Convalescent plasma has no benefit for patients with COVID-19 who are severely ill and in intensive care, according to early findings from the ‘Randomised, Embedded, Multifactorial, Adaptive Platform trial for Community-Acquired Pneumonia’ (REMAP-CAP) trial (12 January 2021);
- No significant differences were observed in clinical status or overall mortality between patients treated with convalescent plasma and those who received placebo (Simonovich et al, 24 November 2020);
- No difference in 28 day mortality or progression to severe disease among patients with moderate COVID-19 treated with convalescent plasma along with best standard of care compared with best standard of care alone (Agarwal et al, 22 October 2020);
- No significant difference in time to clinical improvement within 28 days, mortality or time to hospital discharge in patients treated with convalescent plasma. Trial was terminated early and may have been underpowered to detect a clinically important difference (Li et al, 3 June 2020).
Ongoing trials
- RECOVERY;
- REMAP-CAP;
- Convalescent Plasma to Limit COVID-19 Complications in Hospitalized Patients (CONTAIN COVID-19);
- Passive Immunity Trial Of the Nation for COVID-19 (PassItOnII);
- Convalescent Plasma as Adjunct Therapy for COVID-19 (PlaSenTer);
- Treatment of Patients With COVID-19 With Convalescent Plasma (COOPCOVID-19);
- A Trial of CONvalescent Plasma for Hospitalized Adults With Acute COVID-19 Respiratory Illness (CONCOR-1);
- Evaluating the Efficacy of Convalescent Plasma in Symptomatic Outpatients Infected With COVID-19;
- Inhaled Corticosteroid Treatment of COVID-19 Patients With Pneumonia.
Azithromycin
- Macrolide antibiotic;
- Some in vitro activity against some viruses, such as influenza A and zika;
- May reduce cytokine levels, which can promote inflammation.
Evidence
- Among outpatients with SARS-CoV-2 infection, treatment with a single dose of oral azithromycin compared with placebo did not result in a greater likelihood of being free of symptoms at day 14 (Oldenburg et al, 16 July 2021);
- In patients with mild-to-moderate COVID-19 managed without hospital admission, adding azithromycin to standard care treatment did not reduce the risk of subsequent hospital admission or death (Hinks et al, 9 July 2021);
- Published findings from the PRINCIPLE trial do not justify the routine use of azithromycin for reducing time to recovery or risk of hospitalisation for people with suspected COVID-19 in the community (PRINCIPLE Trial Collaborative Group, 4 March 2021);
- Azithromycin should not be used in the management of confirmed or suspected COVID-19, the Department of Health and Social Care has advised (1 February 2021);
- Preliminary analysis of data from the RECOVERY trial has revealed that there was no significant difference in the primary endpoint of 28-day mortality between azithromycin (19%) and usual care (19%). There was also no evidence of beneficial effects on the risk of progression to mechanical ventilation or length of hospital stay (14 December 2020);
- In patients with severe COVID-19, adding azithromycin to standard of care treatment (which included hydroxychloroquine) did not improve clinical outcomes (Furtado et al, 4 September 2020);
- Among patients hospitalized with mild-to-moderate COVID-19, the use of hydroxychloroquine, alone or with azithromycin, did not improve clinical status at 15 days as compared with standard care (Cavalcanti et al, 23 July 2020).
Ongoing trials
- RECOVERY;
- PRINCIPLE.
Doxycycline
- A broad-spectrum tetracycline-class antibiotic used in the treatment of infections caused by bacteria and certain parasites;
- Considered as a potential treatments for COVID-19 in the community due to its anti-inflammatory, antibacterial and possibly antiviral effects;
- It has been reported that doxycycline lowers significantly proinflammatory cytokines, including interleukin-6.
Evidence
- In patients with suspected COVID-19 in the community in the UK, who were at high risk of adverse outcomes, treatment with doxycycline was not associated with clinically meaningful reductions in time to recovery or hospital admissions or deaths related to COVID-19, and should not be used as a routine treatment for COVID-19 (Butler et al, 27 July 2021);
- Doxycycline should not be used in the management of confirmed or suspected COVID-19, the Department of Health and Social Care has advised (1 February 2021);
- Interim analyses of data from the doxycycline arm of the PRINCIPLE trialconcluded that there was no beneficial effect in patients aged over 50 who are treated with doxycycline at home in the early stages of COVID-19. The researchers also found that the treatment did not reduce the time taken for people to first report that they feel recovered from COVID-19.
Ongoing trials
- PRINCIPLE;
- Doxycycline Ambulatoire COVID-19 (DYNAMIC);
- Safety and Efficacy of Doxycycline and Rivaroxaban in COVID-19 (DOXYCOV);
- DYNAMIC Study (DoxycYcliNe AMbulatoIre COVID-19) (DYNAMIC);
- An Outpatient Clinical Trial Using Ivermectin and Doxycycline in COVID-19 Positive Patients at High Risk to Prevent COVID-19 Related Hospitalization;
- OD-doxy-PNV-COVID-19 Old Drug ” DOXY ” for Prevention of New Virus ” COVID-19 “
Interferons
- Modulate immune response to some viral infections;
- Only limited clinical trial data are currently available on the efficacy of IFNs for treatment of COVID-19;
- Clinical trials are currently evaluating IFN beta-1a, subcutaneous and inhaled, or IFN beta-1b, generally added to antivirals;
- Synairgen is developing a formulation of IFN-beta, called SNG001, for direct delivery to the lungs via nebulisation, to treat and/or prevent lower respiratory tract illness caused by respiratory viruses.
- In clinical trials in asthma and COPD, SNG001 has been well tolerated and shown to upregulate lung antiviral defences. In two phase II trials in asthma and one study in COPD, SNG001 improved lung function in patients with a cold or flu infection.
Evidence
- The results of the ACTIV-2 Outpatient Monoclonal Antibodies and Other Therapies Trial, released on 4 October 2022, showed no statistically significant differences between the SNG001 and placebo arms in the proportion of individuals without detectable nasopharyngeal RNA at days 3, 7, and 14 post-treatment, or the duration of COVID-19 associated symptoms;
- Subcutaneous interferon beta-1a plus remdesivir was not superior to remdesivir alone in hospitalised patients with COVID-19 pneumonia. Patients who required high-flow oxygen at baseline had worse outcomes after treatment with interferon beta-1a compared with those given placebo (Kalil et al, 18 October 2021);
- Interim results from the Solidarity trial suggest that subcutaneous IFN beta-1a has little or no effect on mortality in patients who are hospitalised with COVID-19 (15 October 2020).
- Results of a small randomised, double-blind, placebo-controlled, phase II pilot trial of inhaled nebulised interferon beta-1a (SNG001) in adults admitted to hospital with COVID-19 suggest a greater odds of improvement on the WHO Ordinal Scale for Clinical Improvement (OSCI) (odds ratio 2·32 [95% CI 1·07–5·04]) on day 15 or 16 versus placebo. and were more likely than those receiving placebo to recover to an OSCI score of 1 (no limitation of activities) during treatment (hazard ratio 2·19 [95% CI 1·03–4·69]). (Monk et al, 12 November 2020).
Ongoing trials
- Study to Assess Efficacy and Safety of Inhaled Interferon-β Therapy for COVID-19 (SPRINTER);
- Trial of Inhaled Anti-viral (SNG001) for SARS-CoV-2 (COVID-19) Infection;
- REMAP-CAP;
- Comparing efficacy and safety of inhaled SNG001 to placebo;
- SOLIDARITY;
- DISCOVERY;
- RECOVERY.
Tocilizumab
- Monoclonal antibody that inhibits interleukin-6 (IL-6), which is vital in the immune response to SAR-CoV-2;
- Indicated for treatment of rheumatoid arthritis;
- May combat cytokine release syndrome in severely ill COVID-19 patients.
Evidence
- Results from the REMAP-CAP trial show that among critically ill patients with COVID-19 randomised to receive 1 or more therapeutic interventions, treatment with an IL-6 receptor antagonist had a greater than 99.9% probability of improved 180-day mortality compared with patients randomised to the control, and treatment with an antiplatelet had a 95.0% probability of improved 180-day mortality compared with patients randomised to the control (Writing Committee for the REMAP-CAP Investigators, 16 December 2022);
- A randomised controlled trial found that drugs targeting IL-1 or IL-6 did not shorten the time to clinical improvement in this sample of patients with COVID-19, hypoxic respiratory failure, low Systematic Organ Failure Assessment score, and low baseline mortality risk (Declercq et al, 29 October 2021);
- Tocilizumab plus remdesivir did not shorten time to hospital discharge or “ready for discharge” to day 28 compared with placebo plus remdesivir in patients with severe COVID-19 pneumonia (Rosas et al, 5 October 2021);
- A rapid policy statement, issued jointly by the Department of Health and Social Care (DHSC) and Scottish and Welsh governments on 12 September 2021 propose that tocilizumab and sarilumab should be considered equally as treatment options through routine commissioning for adult patients, aged 18 years and over, who have been hospitalised with COVID-19;
- Follow-up analysis of a randomised clinical trial suggests that tocilizumab may be considered for treating patients with moderate-to-severe COVID-19–associated pneumonia and high CRP levels (Mariette et al, 24 May 2021);
- In hospitalised COVID-19 patients with hypoxia and systemic inflammation, tocilizumab improved survival and other clinical outcomes. These benefits were seen regardless of the amount of respiratory support and were additional to the benefits of systemic corticosteroids (RECOVERY Collaborative Group; 1 May 2021);
- In a randomised trial involving hospitalised patients with severe COVID-19 pneumonia, the use of tocilizumab did not result in significantly better clinical status or lower mortality than placebo at 28 days (Rosas et al, 22 April 2021);
- Roche announces that the global phase III randomised, double-blind, multicentre REMDACTA study of tocilizumab plus remdesivir, versus placebo plus remdesivir, did not meet its primary endpoint. This was measured by improved time to hospital discharge up to day 28 in patients with severe COVID-19 pneumonia receiving standard of care (11 March 2021);
- Routine use of tocilizumab in patients admitted to hospital with moderate to severe COVID-19 is not supported. However, post-hoc evidence suggests tocilizumab might still be effective in patients with severe COVID-19 and so should be investigated further in future studies (Soin et al, 4 March 2021);
- Published results from the REMAP-CAP trial in New England Journal of Medicine (NEJM) show that in critically ill patients with COVID-19 receiving organ support in ICUs, treatment with the IL-6 receptor antagonists tocilizumab and sarilumab improved outcomes, including survival (REMAP-CAP Investigators, 25 February 2021);
- Results from the COVACTA trial in NEJM showed that in hospitalised patients with severe COVID-19 pneumonia, the use of tocilizumab did not result in significantly better clinical status or lower mortality than placebo at 28 days (Rosas et al, 25 February 2021).
- Preliminary results from the RECOVERY trial suggest that for every 25 patients treated with tocilizumab, one additional life would be saved, and for patients who were not on invasive mechanical ventilation, tocilizumab also significantly reduced the chance of progressing to invasive mechanical ventilation or death from 38% to 33% (11 February 2021);
- A randomised controlled trial has found that, in patients with severe or critical COVID-19, tocilizumab plus standard care was not superior to standard care alone in improving clinical outcomes at 15 days and might increase mortality (Veiga et al, 20 January 2021);
- Early results from the REMAP-CAP trial suggest that the mortality rate was 35.8% for patients receiving the current standard of care alone and 27.3%, on average, for patients given tocilizumab or sarilumab; (28.0% for tocilizumab, 22.2% for sarilumab) and NHS guidance has been updated to recommend both drugs are considered in the treatment of COVID-19 patients admitted to intensive care (7 January 2021);
- In hospitalised patients with COVID-19 pneumonia who were not receiving mechanical ventilation, tocilizumab reduced the likelihood of progression to the composite outcome of mechanical ventilation or death, but it did not improve survival. No new safety signals were identified (Salama et al. 17 December 2020);
- Early findings from the REMAP-CAP trial have suggested that tocilizumab significantly improves outcomes for critically ill patients with severe COVID-19, potentially reducing mortality and time spent in intensive care (19 November 2020);
- Tocilizumab was not effective for preventing intubation or death in moderately ill hospitalised patients with COVID-19. Some benefit or harm cannot be ruled out, however, because the confidence intervals for efficacy comparisons were wide (Stone et al, 21 October 2020);
- Tocilizumab may reduce the need for mechanical and noninvasive ventilation or death by day 14 but not mortality by day 28 (Hermine et al, 20 October 2020);
- For hospitalised adult patients with COVID-19 pneumonia and partial pressure of arterial oxygen to fraction of inspired oxygen (Pao2/Fio2) ratio of between 200-300mmHg, tocilizumab had no benefit on disease progression compared with standard care (Salvarani et al, 20 October 2020);
- Patients who received tocilizumab were 44% less likely to progress to mechanical ventilation or death compared to patients who received placebo plus standard of care according to late-stage clinical data (18 September 2020).
Ongoing trials
- ‘Tocilizumab in COVID-19 Pneumonia’ (TOCIVID-19);
- Phase III clinical trial, part of a global effort, to assess whether tocilizumab might have therapeutic value for COVID-19 patients who have developed or at high risk of developing serious lung damage from SARS-CoV-2 infections;
- Low-dose Tocilizumab Versus Standard of Care in Hospitalized Patients With COVID-19 (COVIDOSE-2);
- Trial of Tocilizumab for Treatment of Severe COVID-19: ARCHITECTS (ARCHITECTS).
Sarilumab
- Monoclonal antibody that inhibits IL-6, which is vital in the immune response to SAR-CoV-2;
- Indicated for treatment of rheumatoid arthritis;
- May combat cytokine release syndrome and pulmonary symptoms in severely ill COVID-19 patients.
Evidence
- Results from the REMAP-CAP trial show that among critically ill patients with COVID-19 randomised to receive 1 or more therapeutic interventions, treatment with an IL-6 receptor antagonist had a greater than 99.9% probability of improved 180-day mortality compared with patients randomised to the control, and treatment with an antiplatelet had a 95.0% probability of improved 180-day mortality compared with patients randomised to the control (Writing Committee for the REMAP-CAP Investigators, 16 December 2022);
- A rapid policy statement, issued jointly by the Department of Health and Social Care (DHSC) and Scottish and Welsh governments on 12 September 2021 propose that tocilizumab and sarilumab should be considered equally as treatment options through routine commissioning for adult patients, aged 18 years and over, who have been hospitalised with COVID-19;
- In a randomised controlled, phase III trial of patients with severe or critical COVID-19 who were receiving the local standard of care, there was no observed benefit of intravenous sarilumab over placebo (Lescure et al, 4 March 2021);
- Published results from the REMAP-CAP trial in New England Journal of Medicine show that in critically ill patients with COVID-19 receiving organ support in ICUs, treatment with the IL-6 receptor antagonists tocilizumab and sarilumab improved outcomes, including survival (REMAP-CAP Investigators, 25 February 2021);
- Early results from the REMAP-CAP trial suggest that the mortality rate was 35.8% for patients receiving the current standard of care alone and 27.3%, on average, for patients given tocilizumab or sarilumab; (28.0% for tocilizumab, 22.2% for sarilumab) and NHS guidance has been updated to recommend both drugs are considered in the treatment of COVID-19 patients admitted to intensive care (7 January 2021);
- Sarilumab (Kevzara) demonstrates mixed efficacy results in ongoing COVID-19 trial of hospitalised patients with severe or critical respiratory illness secondary to COVID-19 (27 April 2020);
- Sarilumab fails to meet its primary and key secondary endpoints in US COVID-19 patients requiring mechanical ventilation. Minor positive trends were demonstrated in the primary pre-specified analysis group, but the results did not achieve statistical significance (2 July 2020).
Ongoing trials
- REMAP-CAP.
Regdanvimab
- A monoclonal antibody with activity against SARS-CoV-2;
- Designed to attach to the spike protein of SARS-CoV-2 – when it attaches to the spike protein, the ability of the virus to enter the body’s cells is reduced;
- This is expected to reduce the need for hospitalisation in patients with mild to moderate COVID-19.
Evidence
- Top line results from a phase III trial suggest regdanvimab significantly reduced the risk of hospitalisation or death by 72% for patients at high-risk of progressing to severe COVID-19 up to day 28, compared to placebo. It also significantly reduced the risk of hospitalisation or death by 70% in all patients;
- Following a review of data from an ongoing study looking into the effects of regdanvimab in adult outpatients with COVID-19 symptoms described as mild-to-moderate who do not need supplemental oxygen, the European Medicines Agency’s Committee for Medicinal Products for Human Use concluded that it could be considered a treatment option for patients at high risk of progressing to severe COVID-19.
Canakinumab
- Inhibits interleukin-1 (IL-1), which is vital in the immune response to SAR-CoV-2;
- Indicated to treat certain periodic fever syndromes and gouty arthritis;
- Potential to treat cytokine release syndrome in severely ill COVID-19 patients.
Evidence
- Among patients hospitalised with severe COVID-19, treatment with canakinumab, compared with placebo, did not significantly increase the likelihood of survival without invasive mechanical ventilation at day 29 (Caricchio et al, 20 July 2021);
- An interim analysis of the CAN-COVID trial showed the drug did not meet the primary endpoint of clinical response, defined as survival without the need for mechanical ventilation up to day 29. The drug also failed on a key secondary endpoint, reduction in COVID-19-related death within four weeks after the treatment period.
Anakinra
- Inhibits IL-1, which is vital in the immune response to SAR-CoV-2;
- Indicated for treatment of rheumatoid arthritis, Still’s disease and cryopyrin-associated periodic syndromes;
- Might help to neutralise the cause of acute respiratory distress syndrome (ARDS) among patients with COVID-19;
- Anakinra has been used (off label) for cytokine storm syndromes triggered by other viruses and is reported to be relatively well tolerated, with a favourable safety profile.
Evidence
- On 19 July 2021, the EMA announced that it had started evaluating an application to extend the use of Kineret (anakinra) to include treatment of coronavirus disease 2019 (COVID-19) in adult patients with pneumonia who are at risk of developing severe respiratory failure;
- Anakinra did not improve outcomes in patients with mild-to-moderate COVID-19 pneumonia. Further studies are needed to assess the efficacy of anakinra in other selected groups of patients with more severe COVID-19 (The CORIMUNO-19 collaborative group, 22 January 2021).
Ongoing trials
- REMAP-CAP;
- RECOVERY.
Baricitinib
- Janus-associated tyrosine kinase (JAK) 1 and JAK 2 inhibitor;
- Modulates the immune response by regulating overactive signalling through the Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway;
- Indicated for treatment of rheumatoid arthritis;
- May potentially combat cytokine release syndrome (CRS) in severely ill patients;
- In January 2022, the World Health Organization (WHO) “strongly recommended” baricitinib for patients with severe or critical COVID-19.
Evidence
- Although there was no significant reduction in the frequency of disease progression overall, treatment with baricitinib in addition to standard of care (including dexamethasone) had a similar safety profile to that of standard of care alone, and was associated with reduced mortality in hospitalised adults with COVID-19 (Marconi et al, 1 September 2021);
- Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status among patients with COVID-19, notably among those receiving high-flow oxygen or non-invasive ventilation. The combination was associated with fewer serious adverse events (Kalil et al, 11 December 2020).
Ongoing trials
- RECOVERY (2 February 2021);
- ACTT-II;
- Efficacy of Ramdicivir and Baricitinib for the Treatment of Severe COVID 19 Patients;
- mulTi-Arm Therapeutic Study in Pre-ICu Patients Admitted With Covid-19 – Repurposed Drugs (TACTIC-R);
- Clinical Trial to Evaluate the Efficacy of Different Treatments in Patients With COVID-19.
Ruxolitinib
- Selective inhibitor of JAK 1 and JAK 2;
- Modulates the immune response by regulating overactive signalling through the Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway;
- Indicated for specialist treatments;
- May combat CRS in severely ill patients;
- Currently no known published clinical trial evidence supporting efficacy or safety in patients with COVID-19.
Evidence
- Ruxolitinib 5 mg twice per day showed no benefit in the overall study population. A larger sample is required to determine the clinical importance of trends for increased efficacy in patient subgroups (Han et al, 29 March 2022);
- RUXCOVID trial found that ruxolitinib on top of standard therapy showed no significant reduction in severe complications of COVID-19, including death, respiratory failure requiring mechanical ventilation or admission to the intensive care unit. There was also no relevant benefit for other endpoints including mortality rate by day 29 and time to recovery.
Ongoing trials
Tofacitinib
- An orally administered selective inhibitor of JAK 1 and JAK 3, with functional selectivity for JAK2;
- Also modulates the action of interferons and interleukin-6, decreasing the release of cytokines by type 1 and type 17 helper T cells, which are implicated in the pathogenesis of the acute respiratory distress syndrome (ARDS);
- Thought that the action of tofacitinib may ameliorate progressive, inflammation-driven lung injury in hospitalised patients with COVID-19.
Evidence
- Among patients hospitalised with COVID-19 pneumonia, tofacitinib led to a lower risk of death or respiratory failure through day 28 than placebo (Guimarães et al, 16 June 2021).
Ongoing trials
Acalabrutinib
- Bruton’s tyrosine kinase inhibitor;
- In clinical development for people with chronic lymphocytic leukaemia, approved for this use in the United States;
- Early clinical data have shown it can lead to a decrease in inflammation and reduction in the severity of COVID-19-induced respiratory distress.
Evidence
Ongoing trials
Imatinib
- A tyrosine kinase inhibitor used in the treatment of some types of leukaemia, blood disorders and gastrointestinal stromal tumours;
- Data suggest that imatinib may have anti-SARS-CoV-2 activity, either on-target through inhibition of ABL1/2 or off-target through a previously unrecognised protease-inhibiting effect.
Ongoing trials
- SOLIDARITY;
- Trial of Imatinib for Hospitalized Adults With COVID-19;
- Clinical Trial to Evaluate Efficacy of 3 Types of Treatment in Patients With Pneumonia by COVID-19 (Covid19COVINIB).
Brensocatib
- Reversible inhibitor of the dipeptidyl peptidase-1 enzyme, which is known to be associated with pathogen destruction and inflammatory mediation;
- Not licensed in the UK;
- Could be beneficial for ARDS in severely ill COVID-19 patients.
Ongoing trials
Ravulizumab
- Recombinant monoclonal antibody;
- Used routinely in blood diseases where complement activation destroys red blood cells;
- Potential to treat CRS in severely ill COVID-19 patients.
Ongoing trials
Namilumab
- Human immunoglobulin G1 monoclonal antibody currently in late-stage trials for the treatment of rheumatoid arthritis and ankylosing spondylitis;
- Currently being investigated to see if it can help manage inflammation associated with COVID-19.
Ongoing trials
- CATALYST.
Infliximab
- Chimeric monoclonal antibody indicated to treat inflammatory conditions, including rheumatoid arthritis and inflammatory bowel disease;
- Currently being investigated to see if it can help manage inflammation associated with COVID-19.
Ongoing trials
- SOLIDARITY;
- CATALYST;
- Immune Modulators for Treating COVID-19 (ACTIV-1 IM);
- RECOVERY.
Adalimumab
- An anti-tumour necrosis factor (TNF) drug already used for a wide-range of inflammatory conditions including rheumatoid arthritis and inflammatory bowel disease;
- Recent studies of patients with COVID-19 have shown that patients already taking anti-TNF drugs for other conditions were less likely to be admitted to hospital.
Ongoing trials
Otilimab
- Monoclonal antibody already in trials for the treatment of arthritis;
- May be able to help to block the effects of one of the types of cytokine (known as GM-CSF).
Evidence
- GlaxoSmithKline announced results from the phase II proof of concept OSCAR study with otilimab which showed that in patients of all ages treatment difference was not statistically significant between the otilimab and standard of care groups, but in analysis by age, patients 70 years and older had an increased chance of being alive and free of respiratory failure 28 days after treatment with otilimab compared to the standard care group (65.1% vs 45.9%);
Ongoing trials
Medi3506
- Interleukin-33 monoclonal antibody developed for skin disorders.
Ongoing trials
- ACCORD.
Monoclonal antibody cocktails
- Several companies are developing novel monoclonal antibodies to bind to and neutralise the SARS-CoV-2 virus;
- Monoclonal antibody ‘cocktails’ contain two antibodies and trials will investigate whether the therapy can improve the outcomes for COVID-19 patients;
- The cocktails will also be tested as a preventive therapy in those who are healthy but at high risk of getting sick because they work in a healthcare setting or have been exposed to an infected person;
- Patients in hospitals in England could start receiving neutralising monoclonal antibodies (nMABs) as a treatment for COVID-19 by early October 2021.
REGEN-COV/Ronapreve
- REGEN-COV comprises casirivimab with imdevimab and is authorised for the treatment of mild-to-moderate COVID-19 in adults and paediatric patients with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progressing to severe COVID-19 and/or hospitalisation;
- On 20 July 2021, Japan became the first country to grant full approval for the use of Regeneron’s casirivimab and imdevimab antibody cocktail to treat patients with mild to moderate COVID-19;
- On 20 August 2021, Ronapreve (casivirimab and imdevimab) became the first monoclonal antibody cocktail to be approved by the Medicines and Healthcare products Regulatory Agency (MHRA) for use in the prevention and treatment of acute COVID-19 in the UK;
- On 15 September 2022, the WHO “strongly advises” against casirivimab-imdevimab for COVID-19 patients.
Evidence
- Treatment with subcutaneous casirivimab and imdevimab antibody combination compared with placebo significantly reduced the incidence of symptomatic COVID-19 among recently exposed, asymptomatic individuals (O’Brien et al, 14 January 2022);
- Additional results from a phase III trial suggest that a single dose of REGEN-COV (1,200 mg subcutaneous) reduced the risk of COVID-19 by 81.6% during the pre-specified follow-up period (months 2-8), maintaining the 81.4% risk reduction previously reported during month one;
- REGEN-COV reduced the risk of COVID-19–related hospitalisation or death from any cause, and it resolved symptoms and reduced the SARS-CoV-2 viral load more rapidly than placebo (Weinreich et al, 29 September 2021);
- RECOVERY trial results suggest that REGEN-COV reduces the risk of death and the length of hospital stay for seronegative (patients who had not mounted their own immune response) hospitalised patients with severe COVID-19 (16 June 2021);
- Results from a phase III trial of recently infected asymptomatic COVID-19 patients found that REGEN-COV (casirivimab with imdevimab) decreased the overall risk of patients progressing to symptomatic COVID-19 by 31%; the trial’s primary endpoint. It also decreased the overall risk of progression by 76% after the third day, decreased the duration of symptoms and significantly reduced viral levels (12 April 2021);
- Topline results from a phase III outcomes trial suggest that investigational REGEN-COV (casirivimab with imdevimab) significantly reduced the risk of hospitalisation or death by 70% (1,200 mg intravenous [IV]) and 71% (2,400 mg IV) compared to placebo. It also met all secondary endpoints, including the ability to reduce symptom duration (23 March 2021);
- The European Medicines Agency’s Committee for Medicinal Products for Human Use concludes that REGN-COV2 can be used for the treatment of confirmed COVID-19 in patients who do not require supplemental oxygen and who are at high risk of progressing to severe COVID-19;
- In an interim analysis, the REGN-COV2 antibody cocktail was found to reduce viral load, with a greater effect in patients whose immune response had not yet been initiated or who had a high viral load at baseline. Safety outcomes were similar in the combined REGN-COV2 dose groups and the placebo group (Weinreich et al. 17 December 2020);
- Regeneron Pharmaceuticals, Inc. announces positive, prospective results from an ongoing Phase 2/3 seamless trial in the COVID-19 outpatient setting showing its investigational antibody cocktail, REGN-COV2, met the primary and key secondary endpoints. REGN-COV2 significantly reduced viral load and patient medical visits (28 October 2020).
Ongoing trials
- RECOVERY;
- OPTIMISE-C19;
- COVID-19 Study Assessing the Efficacy and Safety of Anti-Spike SARS CoV-2 Monoclonal Antibodies for Prevention of SARS CoV-2 Infection Asymptomatic in Healthy Adults and Adolescents Who Are Household Contacts to an Individual With a Positive SARS-CoV-2 RT-PCR Assay;
- COVID-19 Study to Assess Immunogenicity, Safety, and Tolerability of Moderna mRNA-1273 Vaccine Administered With Casirivimab+Imdevimab in Healthy Adult Volunteers;
- ‘Safety, Tolerability and Efficacy of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies for Hospitalised Adult Patients with COVID-19’;
- Safety, Tolerability, and Efficacy of Anti-Spike (S) SARS-CoV-2 Monoclonal Antibodies for the Treatment of Ambulatory Adult and Pediatric Patients With COVID-19;
- COVID-19 Study Assessing the Virologic Efficacy of REGN10933+REGN10987 Across Different Dose Regimens in Adult Outpatients With SARS-CoV-2 Infection.
Bamlanivimab plus etesevimab
Evidence
- Among high-risk ambulatory patients, bamlanivimab plus etesevimab led to a lower incidence of COVID-19–related hospitalization and death than did placebo and accelerated the decline in the SARS-CoV-2 viral load (Dougan et al, 14 July 2021);
- Eli Lilly announce new data from the BLAZE-1 Phase III study, demonstrating that bamlanivimab (LY-CoV555) 700mg and etesevimab (LY-CoV016) 1400mg together significantly reduced COVID-19 related hospitalizations and deaths (“events”) in high-risk patients recently diagnosed with COVID-19 (10 March 2021);
- Treatment with bamlanivimab and etesevimab combination therapy, but not bamlanivimab monotherapy, resulted in a reduction in SARS-CoV-2 log viral load at day 11 in patients with mild to moderate COVID-19 (Gottlieb et al, 21 January 2021).
Ongoing trials
- OPTIMISE-C19.
AZD7442/Evusheld
- AZD7442/Evusheld is a combination of two long-acting monoclonal antibodies (LAABs) – tixagevimab and cilgavimab – derived from convalescent patients after SARS-CoV-2 infection;
- LAABs mimic natural antibodies and have the potential to treat and prevent disease progression in patients already infected with the virus, as well as to be given as a preventative intervention prior to exposure to the virus;
- In pre-clinical experiments the two LAABs have been shown to block the binding of the SARS-CoV-2 virus to host cells and protect against infection in cell and animal models of disease;
- On 14 October 2021, the EMA announced that it was commencing a rolling review of the evidence around AZD7442;
- On 17 March 2022, Evusheld became the first monoclonal antibody treatment to be authorised by the MHRA for the prevention of COVID-19 as a pre-exposure prophylaxis.
Evidence
- Among patients hospitalised with COVID-19 receiving remdesivir and other standard care, tixagevimab–cilgavimab did not improve the primary outcome of time to sustained recovery but was safe and mortality was lower (ACTIV-3–Therapeutics for Inpatients with COVID-19 (TICO) Study Group, 8 July 2022);
- A single intramuscular tixagevimab–cilgavimab dose provided statistically and clinically significant protection against progression to severe COVID-19 or death versus placebo in unvaccinated individuals and safety was favourable. Treating mild to moderate COVID-19 earlier in the disease course with tixagevimab–cilgavimab might lead to more favourable outcomes (Montgomery et al, 7 June 2022);
- A single dose of AZD7442 had efficacy for the prevention of COVID-19, without evident safety concerns; extended follow-up at a median of 6 months showed a relative risk reduction of 82.8% (Levin et al, 20 April 2022);
- Results from the PROVENT study suggested that Evusheld reduced the risk of people developing any COVID-19 symptoms by 77% and was well-tolerated. This is the first long-acting monoclonal antibody combination that represents a potential new option to augment COVID-19 prevention (Levin et al, 4 December 2021).
Ongoing trials
- ACTIV-3: Therapeutics for Inpatients With COVID-19 (TICO);
- Phase III Double-blind, Placebo-controlled Study of AZD7442 for Post- Exposure Prophylaxis of COVID-19 in Adults (STORM CHASER);
- Phase III Double-blind, Placebo-controlled Study of AZD7442 for Pre-exposure Prophylaxis of COVID-19 in Adult. (PROVENT)
BRII-196/BRII-198
- Two monoclonal antibodies isolated from COVID-19 survivors that bind distinct and complementary epitopes of the SARS-CoV-2 spike protein;
- Potently inhibit SARS-CoV-2 replication and have shown efficacy among outpatients with COVID-19 for preventing disease progression to death or hospitalisation.
Evidence
- Neither sotrovimab nor BRII-196 plus BRII-198 showed efficacy for improving clinical outcomes among adults hospitalised with COVID-19 (ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group, 23 December 2021).
Ongoing trials
- ACTIV-2: A Study for Outpatients With COVID-19;
- ACTIV-3: Therapeutics for Inpatients With COVID-19 (TICO).
Bamlanivimab (monotherapy)
- Bamlanivimab (LY-CoV555) is a potent neutralising IgG1 monoclonal antibody directed against the spike protein on SARS-CoV-2;
- Designed to block viral attachment and entry into human cells, thus neutralising the virus, potentially preventing and treating COVID-19.
Evidence
- Among 314 participants (163 receiving bamlanivimab and 151 placebo), the median time to sustained recovery was 19 days and did not differ between the bamlanivimab and placebo groups. Further independent trials needed (ACTIV-3/TICO Bamlanivimab Study Group, 21 December 2021);
- A randomised phase III clinical trial including 966 participants, concluded that bamlanivimab monotherapy compared with placebo reduced the risk of COVID-19 in residents and staff of skilled nursing and assisted living facilities (Cohen et al, 3 June 2021);
- Initial results from the BLAZE-2 trial suggest that nursing home residents randomised to bamlanivimab have up to an 80% lower risk of contracting COVID-19 versus residents in the same facility randomised to placebo (21 January 2021);
- Treatment with bamlanivimab and etesevimab combination therapy, but not bamlanivimab monotherapy, resulted in a reduction in SARS-CoV-2 log viral load at day 11 in patients with mild to moderate COVID-19 (Gottlieb et al, 21 January 2021);
- Interim analysis of the BLAZE-1 trial found that one of three doses (2800mg) of the neutralizing antibody, bamlanivimab, appeared to accelerate the natural decline in viral load over time in patients with mild or moderate COVID-19, whereas the other doses (700mg and 7000mg) administered had not by day 11 (Chen et al, 28 October 2020);
- The ACTIV-3 trial, which is evaluating multiple investigational agents in hospitalised patients with COVID-19, stopped randomising patients to treatment with LY-CoV555 based on an analysis suggesting that the antibody was not beneficial in this population (26 October 2020).
Ongoing trials
- A Study to Assess if a Medicine Called Bamlanivimab is Safe and Effective in Reducing Hospitalization Due to COVID-19 (B-EPIC);
- OPTIMISE-C19;
- A Study of Immune System Proteins in Participants With Mild to Moderate COVID–19 Illness (BLAZE-4);
- A Study of LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) in Participants With Mild to Moderate COVID-19 Illness (BLAZE-1);
- ACTIV-2: A Study for Outpatients With COVID-19;
- ACTIV-3: Therapeutics for Inpatients With COVID-19 (TICO)
Etesevimab (monotherapy)
- Etesevimab (LY-CoV016) is a recombinant human monoclonal neutralising antibody;
- Targets the SARS-CoV-2 spike protein and blocks binding of the virus to the ACE2 host cell surface receptor;
- Effective for both prophylactic and therapeutic venues against SARS-CoV-2 infection in rhesus macaques.
Ongoing trials
- BLAZE-4;
- BLAZE-1;
- OPTIMISE-C19.
Sotrovimab
- Sotrovimab (VIR-7831) is an investigational dual-action SARS-CoV-2 monoclonal antibody;
- Preclinical data suggest it has the potential to both block viral entry into healthy cells and clear infected cells;
- The antibody binds to an epitope on SARS-CoV-2 that is shared with SARS-CoV-1 (the virus that causes SARS), indicating that the epitope is highly conserved, which may make it more difficult for resistance to develop;
- On 18 November 2021, the European Medicines Agency announced that it had started evaluating an application for marketing authorisation for sotrovimab;
- On 2 December 2021, the Medicines and Healthcare products Regulatory Agency (MHRA) approved sotrovimab for people with mild-to-moderate COVID-19 and at least one risk factor for developing severe illness;
- On 15 September 2022, the WHO “strongly advises” against sotrovimab for COVID-19 patients.
Evidence
- Findings from a randomised clinical trial support sotrovimab as a treatment option for non-hospitalised, high-risk patients with mild to moderate COVID-19, although efficacy against SARS-CoV-2 variants that have emerged since the study was completed is unknown (Gupta et al, 14 March 2022);
- Neither sotrovimab nor BRII-196 plus BRII-198 showed efficacy for improving clinical outcomes among adults hospitalised with COVID-19 (ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group, 23 December 2021);
- Interim analysis of an ongoing phase 3 trial found that, among high-risk patients with mild-to-moderate COVID-19, sotrovimab reduced the risk of disease progression (Gupta et al, 27 October 2021);
- The European Medicines Agency starts a rolling review into VIR-7831 based on preliminary results from an ongoing study looking at the ability of the medicine to prevent hospitalisation or death in non-hospitalised patients with COVID-19 (7 May 2021);
- An interim analysis of data from 583 patients enrolled in the COMET-ICE trial, demonstrated an 85% (p=0.002) reduction in hospitalisation or death in patients receiving VIR-7831 as monotherapy compared to placebo (10 March 2021).
Ongoing trials
- VIR-7831 for the Early Treatment of COVID–19 in Outpatients (COMET-ICE);
- Safety, Tolerability and Pharmacokinetics of Second Generation VIR-7831 Material in Non-hospitalized Participants With Mild to Moderate COVID-19 (COMET-PEAK);
- TICO;
- BLAZE-4;
- Intramuscular VIR-7831 (Sotrovimab) for Mild/Moderate COVID-19;
- OPTIMISE-C19.
Leronlimab
- An investigational humanised monoclonal antibody targeted against the CCR5 receptor, which appears to play a central role in modulating immune cell trafficking to sites of inflammation;
- Being looked at as a potential therapy in the treatment of triple negative breast cancer and HIV infection as well as COVID-19.
Ongoing trials
- Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19);
- Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19).
Risankizumab
- Risankizumab is an anti-IL-23 monoclonal antibody developed by Boehringer Ingelheim and AbbVie;
- Being investigated for the treatment of multiple inflammatory diseases, including psoriasis, Crohn’s disease, ulcerative colitis, atopic dermatitis and psoriatic arthritis;
- Approved in the US in 2019 for the treatment of severe plaque psoriasis;
- Will be tested in conjunction with the antiviral drug remdesivir, compared to a placebo plus remdesivir.
Ongoing trials
Lenzilumab
- Investigational recombinant monoclonal antibody targeting human GM-CSF, which is thought to have a role in the pathogenesis of COVID-19–related immune hyper-response;
- Currently being tested in a phase III COVID-19 study and in a phase 1b/2 study as sequenced therapy with CAR-T treatments;
- On 9 July 2021, the MHRA accepted Humanigen’s submission of Lenzilumab for Marketing Authorisation in COVID-19 for expedited rolling review.
Evidence
- Lenzilumab significantly improved survival without invasive mechanical ventilation in hospitalised patients with COVID-19, with a safety profile similar to that of placebo. The added value of lenzilumab beyond other immunomodulators used to treat COVID-19 alongside steroids remains unknown (Temesgen et al, 1 December 2021).
Ongoing trials
- COVID-19 Prevention and Treatment in Cancer; a Sequential Multiple Assignment Randomised Trial; (C-SMART);
- ACTIV-5 / Big Effect Trial (BET-B) for the Treatment of COVID-19.
IMU-838
- IMU-838 is a next-generation selective immune modulator that inhibits the intracellular metabolism of activated immune cells by blocking the enzyme, dihydroorotate dehydrogenase;
- Investigational drug under development as an oral tablet formulation for the treatment of relapsing-remitting multiple sclerosis, inflammatory bowel disease and other chronic inflammatory and autoimmune diseases;
- Also being investigated as a potential treatment option for COVID-19.
Ongoing trials
CD24Fc
- An anti-inflammatory therapy that suppresses production of inflammatory cytokines;
- Might be most effective in patients with a heightened inflammatory response;
- CD24Fc has shown promise as a COVID-19 therapy with systemic effects that might persist against emerging viral variants.
Evidence
- CD24Fc is generally well tolerated and accelerates clinical improvement of hospitalised patients with COVID-19 who are receiving oxygen support. These data suggest that targeting inflammation in response to tissue injuries might provide a therapeutic option for patients hospitalised with COVID-19 (Welker et al, 11 March 2022).
Other or multiple mechanisms
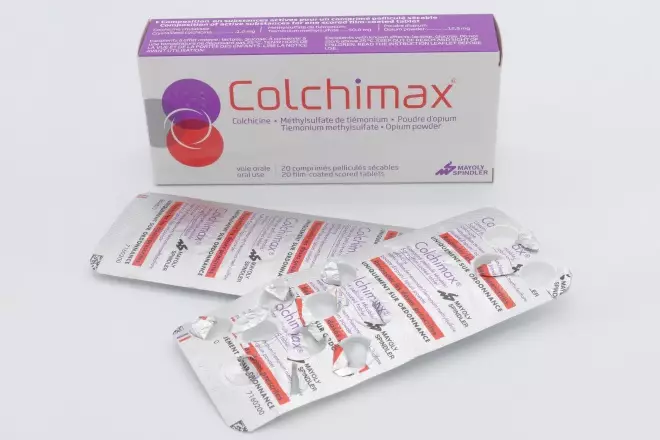
Shutterstock.com
Colchicine
- Medicine for treating inflammation and pain in conditions such as gout;
- Could help ameliorate COVID-19 complications, but there is minimal anecdotal experience and clinical trial data reported to date in COVID-19.
Evidence
- On 13 July 2022, the WHO issues strong advice against colchicine based on data from seven RCTs involving 16,484 patients;
- A randomised clinical trial found that colchicine did not significantly reduce the need for mechanical ventilation or 28-day mortality in patients hospitalised with COVID-19 pneumonia (Diaz et al, 29 December 2021);
- In adults hospitalised with COVID-19, colchicine was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death (RECOVERY Collaborative Group, 18 October 2021);
- The National Institute for Health and Care Excellence (NICE) issues ‘strong recommendation’ that colchicine should not be offered to people in hospital to treat COVID-19 (27 May 2021);
- In community-treated patients including those without a mandatory diagnostic test, the effect of colchicine on COVID-19-related clinical events was not statistically significant. Among patients with PCR-confirmed COVID-19, colchicine led to a lower rate of the composite of death or hospital admission than placebo (Tardif et al, 27 May 2021);
- Preprint from the RECOVERY trial suggest that in adults hospitalised with COVID-19, colchicine was not associated with reductions in 28-day mortality, duration of hospital stay, or risk of progressing to invasive mechanical ventilation or death (Horby et al, 18 May 2021);
- After reviewing the available safety and efficacy data for the colchicine arm the Data Monitoring Committee for the RECOVERY trial saw no convincing evidence that further recruitment would provide conclusive proof of worthwhile mortality benefit either overall or in any pre-specified subgroup (5 March 2021);
- A COVID-19 Therapeutic Alert published by the Department of Health and Social Care on 12 February 2021 states that colchicine should not be used in the management of COVID-19 positive patients other than in the context of a trial, or unless there is an additional licensed indication for its use;
- A small study suggests that colchicine reduced the length of both supplemental oxygen therapy and hospitalisation. The drug was safe and well tolerated. It was not possible to ensure that colchicine reduced mortality of COVID-19 (Lopes et al. 4 February 2021);
- Early results from the COLCORONA trial have shown that the use of colchicine was associated with reductions in the risk of death or hospitalisation compared to placebo; in patients with a proven diagnosis of COVID-19, colchicine reduced hospitalisations by 25%, the need for mechanical ventilation by 50%, and deaths by 44% (23 January 2021);
- Participants who received colchicine had statistically significant improved time to clinical deterioration compared with a control group that did not receive colchicine. However, the authors said that the findings should be considered only hypothesis-generating, given the low enrolment and event rates (Deftereos et al, 24 June 2020).
Ongoing trials
- ANTICOV;
- PRINCIPLE (3 March 2021);
- RECOVERY (27 November 2020; recruitment closed 5 March 2021);
- COLCORONA;
- ‘Colchicine Counteracting Inflammation in COVID-19 Pneumonia’ (COLCOVID-19);
- Colchicine in COVID-19: a Pilot Study (COLVID-19);
- Treatment for Moderate/Severe COVID-19 in a Fragile and Vulnerable Population, Admitted to a Geriatric Hospital Unit or in a Transicional Care Center;
- Colchicine/Statins for the Prevention of COVID-19 Complications (COLSTAT) Trial;
- Anti-Coronavirus Therapies to Prevent Progression of Coronavirus Disease 2019 (COVID-19) Trial (ACTCOVID19).
Dimethyl fumarate
- An immunomodulatory drug thought to prevent NLRP3 inflammasome activation and the process of inflammatory cell death through its action on the protein, gasdermin D;
- Licensed to treat relapsing remitting multiple sclerosis and plaque psoriasis as a long-term immunomodulatory agent and is generally well-tolerated with no major safety concerns;
- Has demonstrated anti-viral and anti-inflammatory effects against SARS-CoV-2 in vitro.
Ongoing trials
- RECOVERY
Angiotensin-converting-enzyme inhibitors/angiotensin II receptor blockers
- Indicated for the treatment of hypertension and heart failure;
- There have been suggestions that the drugs can increase both the risk of infection and the severity of SARS-CoV2, but data are lacking;
- May also have a protective effect against lung damage.
Evidence
- Results from a multicentre blinded randomised controlled trial for outpatients with mild symptomatic COVID-19 disease, losartan did not reduce hospitalisations, although assessment was limited by low event rate. Viral load was not statistically affected by treatment (Puskarich et al, 17 June 2021);
- Severity of COVID-19 is not associated with the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers;
- The BRACE CORONA trial, which tested temporarily stopping an ACE inhibitor/ARB for 30 days versus continuing ACE inhibitors/ARBs in patients who were taking these medications chronically and were hospitalised with a confirmed diagnosis of COVID-19, found no clinical difference, suggesting the medication should generally be continued for those with an indication (Lopes et al, presented at the European Society of Cardiology Congress 2020 on 1 September 2020).
Ongoing trials
- Controlled evaLuation of Angiotensin Receptor Blockers for COVID-19 respIraTorY Disease (CLARITY);
- Switch of Renin-Angiotensin System Inhibitors in Patients With Covid-19 (SWITCH-COVID);
- Angiotensin Receptor Blockers and Angiotensin-converting Enzyme Inhibitors and Adverse Outcomes in Patients With COVID19 (BRACE-CORONA);
- The COVID-RASi Trial (COVID-19),
Statins
- Indicated for the treatment of cardiovascular disease;
- Decrease inflammation, reduce blood clots, and prevent damage to endothelial tissue;
- Some evidence they can act as antivirals;
- Could potentially combat CRS in severely ill patients, but concrete data are lacking.
Ongoing trials
- REMAP-CAP;
- HEAL-COVID (atorvastatin);
- Colchicine/Statins for the Prevention of COVID-19 Complications (COLSTAT) Trial (COLSTAT);
- Atorvastatin as Adjunctive Therapy in COVID-19 (STATCO19);
- ‘Preventing Cardiac Complication of COVID-19 Disease With Early Acute Coronary Syndrome Therapy: A Randomised Controlled Trial’ (C-19-ACS).
Aspirin
- Triple effect of inhibiting virus replication, anticoagulation and anti-inflammatory.
Evidence
- Among critically ill patients with COVID-19, there was a low likelihood that treatment with an antiplatelet agent provided improvement in organ support–free days within 21 days (REMAP-CAP Writing Committee for the REMAP-CAP Investigators, 22 March 2022);
- Among symptomatic clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome. However, the study was terminated after enrollment of 9% of participants because of an event rate lower than anticipated (Connors et al, 11 October 2021);
- Unpublished results from the ‘Randomised Evaluation of COVID-19 Therapy’ (RECOVERY) trial have shown that aspirin does not improve survival for patients hospitalised with COVID-19 (9 June 2021).
Ongoing trials
- REMAP-CAP;
- C-19-ACS;
- ‘Protective Effect of Aspirin on COVID-19 Patients’ (PEAC);
- RECOVERY.
Clopidogrel
- Anti-platelet drug that could help prevent blood-clots associated with COVID-19.
Evidence
- Results from the REMAP-CAP trial show that among critically ill patients with COVID-19 randomised to receive 1 or more therapeutic interventions, treatment with an IL-6 receptor antagonist had a greater than 99.9% probability of improved 180-day mortality compared with patients randomised to the control, and treatment with an antiplatelet had a 95.0% probability of improved 180-day mortality compared with patients randomised to the control (Writing Committee for the REMAP-CAP Investigators, 16 December 2022).
Ongoing trials
- C-19-ACS.
Anticoagulants
- Potential role of anticoagulation in specific COVID-19 patients for improved mortality.
Evidence
- Findings from a randomised controlled trial of 562 patients did not support the addition of a P2Y12 inhibitor to a therapeutic dose of heparin among non–critically ill patients hospitalised for COVID-19 (Berger et al, 18 January 2022);
- Anticoagulation with heparin could increase survival and reduce the need for organ support in patients hospitalised with moderate COVID-19, compared to usual care, results from three integrated platform trials – REMAP-CAP, ACTIV-4 and ATTACC – have suggested. In contrast, anticoagulation therapy did not improve outcomes if started when patients were already critically ill with COVID-19 (The ATTACC, ACTIV-4a, and REMAP-CAP Investigators, 4 August 2021);
- Results of the INSPIRATION randomised clinical trial including 562 patients do not support routine empirical use of intermediate-dose prophylactic anticoagulation in unselected patients with COVID-19 admitted to the ICU (INSPIRATION investigators, 18 March 2021);
- Early initiation of prophylactic anticoagulation compared with no anticoagulation among patients admitted to hospital with COVID-19 was associated with a decreased risk of 30 day mortality and no increased risk of serious bleeding events (Rentsch et al. 11 February 2021).
Ongoing trials
- HEAL-COVID (apixaban);
- REMAP-CAP;
- Effect of the Use of Anticoagulant Therapy During Hospitalization and Discharge in Patients With COVID-19 Infection;
- ‘Weight-Adjusted versus Fixed Low Doses of Low-Molecular-Weight Heparin for Venous Thromboembolism Prevention in COVID-19’ (COVI-DOSE);
- ‘Antithrombotic Therapy to Ameliorate Complications of COVID-19’ (ATTACC);
- ‘Preventing COVID-19 Complications with Low- and High-Dose Anticoagulation’ (COVID-HEP);
- ‘Effect of Anticoagulation Therapy on Clinical Outcomes in COVID-19’ (COVID-PREVENT);
- C-19-ACS.
Bemcentinib
- Selectively inhibits AXL kinase, which blocks viral entry and enhances the antiviral type I IFN response;
- Investigational treatment for COVID-19;
- Reported to exhibit potent anti-viral activity in pre-clinical models against several enveloped viruses, including Ebola and Zika virus.
Evidence
Ongoing trials
Omeprazole
- Proton-pump inhibitor indicated for the treatment of gastroesophageal reflux disease (GORD);
- Being investigated as an additive treatment for COVID-19.
Ongoing trials
- C-19-ACS.
Famotidine
- Histamine-2 receptor antagonist used in the treatment of GORD;
- Some evidence to suggest it is associated with improved patient-reported outcomes in non-hospitalised patients with COVID-19.
Ongoing trials
- Cetirizine and Famotidine for COVID-19;
- An Outpatient Study Investigating Non-prescription Treatments for COVID-19 (PROFACT-01).
Zilucoplan
- Synthetic macrocyclic peptide inhibitor already in trial for potential treatment of the skeletomuscular disorder myasthenia gravis;
- Could reduce damage to lung tissue caused by the virus.
Ongoing trials
- ACCORD.
Ascorbic acid/vitamin C
- Use of vitamin C could be effective in terms of mortality and secondary outcomes in patients with COVID-19 pneumonia due to its anti-inflammatory and antioxidant properties.
Evidence
- Evidence from a randomised clinical trial of 214 patients suggests that treatment with zinc, ascorbic acid, or both does not affect SARS-CoV-2 symptoms (Thomas et al. 12 February 2021).
Ongoing trials
- REMAP-CAP;
- The Effect of Melatonin and Vitamin C on COVID-19;
- International ALLIANCE Study of Therapies to Prevent Progression of COVID-19;
- Vitamin D, Omega-3, and Combination Vitamins B, C and Zinc Supplementation for the Treatment and Prevention of COVID-19;
- A Study of Hydroxychloroquine, Vitamin C, Vitamin D, and Zinc for the Prevention of COVID-19 Infection (HELPCOVID-19);
- ‘Lessening Organ Dysfunction with Vitamin C (LOVIT);
- ‘Use of Ascorbic Acid in Patients with COVID 19’.
Vitamin D3
- Vitamin D insufficiency is a potential risk factor for non-communicable and acute respiratory tract diseases, including viral infections;
- It has been speculated that optimal serum levels of vitamin D may have immunomodulatory and anti-inflammatory properties, and could possibly benefit patients with COVID-19.
Evidence
- Among hospitalized patients with COVID-19, a single high dose of vitamin D3, compared with placebo, did not significantly reduce hospital length of stay. The findings do not support the use of a high dose of vitamin D3 for treatment of moderate to severe COVID-19 (Murai et al, 17 February 2021).
Ongoing trials
- HELPCOVID-19;
- Vitamin D Status and Immune-inflammatory Status in Different UK Populations With COVID-19 Infection;
- Trial of Vitamin D to Reduce Risk and Severity of COVID-19 and Other Acute Respiratory Infections (CORONAVIT);
- The VITDALIZE Study: Effect of High-dose Vitamin D3 on 28-day Mortality in Adult Critically Ill Patients (VITDALIZE);
- Vitamin D and COVID-19 Trial (VIVID).
Aviptadil
- Synthetic form of human vasoactive intestinal peptide;
- Indicated for treatment of erectile dysfunction;
- Reduces inflammation in the lungs and protects the alveolar type II cells that are believed to be an entry route for the SARS-CoV-2 to invade the lungs.
Ongoing trials
- ACTIV-3b;
- An Adaptive Platform Trial for Critically Ill Patients (I-SPY_COVID);
- ‘Inhaled Aviptadil for the Treatment of Non-Acute Lung Injury in COVID-19’ (AVINALI).
Tradipitant
- A neurokinin-1 receptor antagonist that works by blocking substance P, a neuropeptide secreted by neuronal cells and inflammatory cells that has multiple effects in different tissues;
- Currently in clinical development for gastroparesis, motion sickness and atopic dermatitis.
Evidence
Ongoing trials
Nitric oxide
- Nitric oxide (NO) inhalation has been used as a pulmonary vasodilator and has been found to have antiviral activity against other coronavirus strains;
- Preliminary data support a microbicidal effect of high concentration inhaled NO;
- NO is being investigated to see if it can prevent the deterioration to a severe form of COVID-19 when administered at an early stage of the disease.
Ongoing trials
- A Study to Assess Efficacy and Safety of RESP301 Plus Standard of Care (SOC) Compared to SOC Alone in Hospitalized Participants With COVID-19;
- PREvent Viral Exposure aNd Transmission Study: a SARS-CoV-2 PEP Study (PREVENT);
- Nitric Oxide Inhalation Therapy for COVID-19 Infections in the ED (NO COV-ED);
- Inhaled Nitric Oxide Gas Therapy in Mechanically Ventilated Patients with Severe Acute Respiratory Syndrome in COVID-19.
Fluvoxamine
- Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) used primarily for the treatment of obsessive–compulsive disorder;
- It is thought that fluvoxamine may prevent clinical deterioration in patients with COVID-19 by stimulating the σ-1 receptor, which regulates cytokine production.
Evidence
- In a randomized trial of metformin, ivermectin, and fluvoxamine none prevented the occurrence of hypoxemia, an emergency department visit, hospitalisation, or death associated with COVID-19 (Bramante et al, 18 August 2022);
- On 13 July 2022, the WHO advises against the use of fluvoxamine except in clinical trials, this was informed by data from three randomised controlled trials involving over 2,000 patients, it said;
- A meta-analysis of the available randomised clinical trial evidence for fluvoxamine in the outpatient management of COVID-19 suggested that fluvoxamine, a widely available and inexpensive treatment for outpatients with COVID-19, was associated with a reduction in hospitalisations (Lee et al, 6 April 2022);
- Treatment with fluvoxamine (100 mg twice daily for 10 days) among high-risk outpatients with early diagnosed COVID-19 reduced the need for hospitalisation defined as retention in a COVID-19 emergency setting or transfer to a tertiary hospital (Reis et al, 27 October 2021);
- Results from the TOGETHER trial, published as a pre-print on 26 August 2021, suggest that treatment with fluvoxamine (100mg twice daily for 10 days) among high-risk outpatients with early diagnosed COVID-19, reduced the need for extended emergency room observation or hospitalisation;
- In a small preliminary study, adult outpatients with symptomatic COVID-19 treated with fluvoxamine, compared with placebo, had a lower likelihood of clinical deterioration over 15 days; however, determination of clinical efficacy would require larger randomized trials with more definitive outcome measures (Lenze et al. 12 November 2020).
Ongoing trials
- TOGETHER trial;
- Outpatient Treatment of SARS-CoV-2 With Ivermectin, Fluvoxamine, and Metformin (COVID-19);
- Repurposed Approved and Under Development Therapies for Patients With Early-Onset COVID-19 and Mild Symptoms;
- ACTIV-6: COVID-19 Study of Repurposed Medications.
Proxalutamide
- A non-steroidal anti-androgen developed for the treatment of prostate and breast cancer.
Evidence
- Topline results from an investigator-initiated trial in Brazil show that treatment with proxalutamide cut mortality risk by 92% and significantly shortened the median length of hospital stay by nine days compared with standard of care in hospitalized patients with COVID-19.
Ongoing trials
- Proxalutamide Treatment for COVID-19 Patients in Intensive Care Unit;
- Proxalutamide (GT0918) Treatment for Outpatients With Mild or Moderate COVID-19 Illness.
Ruconest
- A powder that is made up into a solution for injection; contains the active substance conestat alfa, a copy of the C1 esterase inhibitor protein that occurs naturally in the body;
- Used in patients with hereditary angioedema that is linked to naturally low levels of a protein called C1 esterase inhibitor;
- Studies are seeking to identify if the administration of ruconest can control or stop the systemic hyperinflammation syndrome or cytokine storm in COVID-19.
Ongoing trials
- Prevention of Severe SARS-CoV-2 Infection in Hospitalized Patients With COVID-19;
- Conestat Alfa in the Prevention of Severe SARS-CoV-2 Infection in Hospitalized Patients With COVID-19.
TRV027
- An angiotensin II receptor type 1 (AT1 receptor) selective agonist;
- Fights disruption within the renin-angiotensin system (RAS) by attaching to and rebalancing AT1 receptor activation;
- Currently being investigated by multiple institutions as a potential treatment for acute lung injury contributing to ARDS and abnormal blood clotting in COVID-19 patients.
Evidence
Ongoing trials
- REMAP-CAP COVID-19 ACE2 RAS Modulation Domain trial (launched on 16 March 2021);
- ACTIV-4d RAAS.
Ciclesonide
- A corticosteroid used in inhaled form to treat asthma and intranasally to treat allergic rhinitis;
- Potent antiviral effect when specifically screened for activity against SARS-CoV-2;
- On the basis of in vitro data and the known anti-inflammatory effect of ciclesonide, it has been hypothesised that ciclesonide administered early in the course of COVID-19 could decrease symptom burden in adults presenting with prominent respiratory symptoms.
Evidence
- The results of a randomised clinical trial demonstrated that ciclesonide did not achieve the primary efficacy end point of reduced time to alleviation of all COVID-19–related symptoms (Clemency et al, 22 November 2021);
- Compared with placebo, the combination of inhaled and intranasal ciclesonide did not show a statistically significant increase in resolution of symptoms among healthier young adults with COVID-19 presenting with prominent respiratory symptoms. Further research is needed (Ezer et al, 2 November 2021).
Ongoing trials
- Inhaled Ciclesonide for Outpatients With COVID19 (CONTAIN);
- Inhalation of Ciclesonide for Patients With COVID-19: A Randomised Open Treatment Study (HALT COVID-19) (HALT);
- Early Treatment of Vulnerable Individuals With Non-Severe SARS-CoV-2 Infection (COVERAGE-A);
- Trial of COVID-19 Outpatient Treatment in Individuals With Risk Factors for Aggravation (COVERAGEFrance).
Sabizabulin
- Oral, novel microtubule disruptor;
- Dual antiviral and anti-inflammatory activities in pre-clinical models;
- Has been observed to reduce deaths, respiratory failure, days in the intensive care unit (ICU), and days on mechanical ventilation in people with moderate to severe COVID-19 in phase II trials.
Evidence
- Sabizabulin treatment resulted in a 24.9% absolute reduction in deaths compared with placebo in hospitalized patients with moderate to severe COVID-19 at high risk for ARDS and death, with a lower incidence of adverse and serious adverse events compared with placebo (Barnette et al, 6 July 2022).
Ongoing trials
N-acetylcysteine
- Acetylcysteine is a mucolytic. It is usually given by inhalation but may be given in other ways in a hospital;
- Used for certain lung conditions when increased amounts of mucus make breathing difficult; acetylcysteine liquefies or dissolves mucus so that it may be coughed up.
Evidence
- NAC was beneficial in reducing the mortality rate in patients with COVID-19 and inflammatory parameters, and a reduction in the development of severe respiratory failure; however, it did not affect the length of hospital stay or the need for ICU admission (Panahi et al, 10 Dec 2022).
This article will be updated regularly as more information emerges. If there are any trials or treatments we have missed, let us know.
Clearing the COVID-19 backlog
For views from pharmacists and nurses on the impact of COVID-19 on cancer care and treatment, see: Clearing the COVID-19 patient treatment backlog through advancing efficient and safe delivery of care (promotional content)
Other sources of information:
- American Society of Health-System Pharmacists: https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx?la=en&hash=B414CC64FD64E1AE8CA47AD753BA744EDF4FFB8C
- National Institute of Health Research: https://www.nihr.ac.uk
- ClinicalTrials.gov: https://clinicaltrials.gov
- EU Clinical Trials Register: https://www.clinicaltrialsregister.eu
- 1Treatments and vaccines for COVID-19 . European Medicines Agency. 2020.https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines-covid-19 (accessed 2021).
2 comments
You must be logged in to post a comment.



Thank you for the useful information!
https://clinosol.com/
tenofovir 300 mg is utilized alongside different drugs to treat mortal immunodeficiency disease in grown-ups and youngsters 2 years old and more chronic. It is additionally used to dine continuous HBV in grown-ups and children 2 years old and more seasoned gauging 22 pounds or more.