Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus
Source: Alisdair Macdonald
Download the full print version of the infographic here.
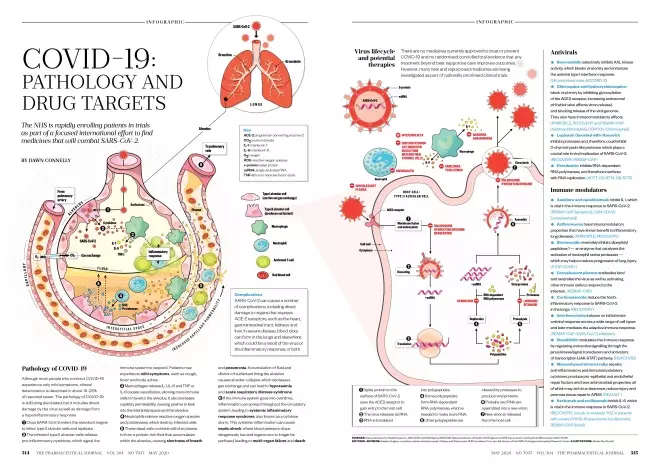
Pathology of COVID-19
Although most people who contract COVID-19 experience only mild symptoms, clinical deterioration is described in about 15–25% of reported cases. The pathology of COVID-19 is still being elucidated, but it includes direct damage by the virus as well as damage from a hyperinflammatory response.
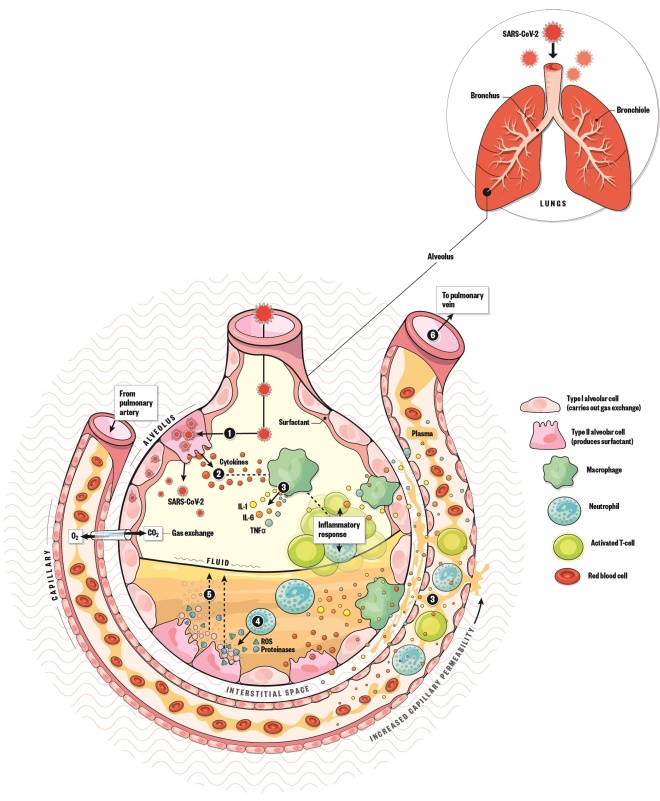
- Once SARS-CoV-2 enters the alveolus it begins to infect type II alveolar cells and replicate.
- The infected type II alveolar cells release pro-inflammatory cytokines, which signal the immune system to respond. Patients may experience mild symptoms, such as cough, fever and body aches.
- Macrophages release IL-1, IL-6 and TNF-α. IL-6 causes vasodilation, allowing more immune cells to travel to the alveolus. It also increases capillary permeability, causing plasma to leak into the interstitial space and the alveolus.
- Neutrophils release reactive oxygen species and proteinases, which destroy infected cells.
- These dead cells combine with the plasma to form a protein-rich fluid that accumulates within the alveolus, causing shortness of breath and pneumonia. Accumulation of fluid and dilution of surfactant lining the alveolus causes alveolar collapse, which decreases gas exchange and can lead to hypoxaemia and acute respiratory distress syndrome.
- If the immune system goes into overdrive, inflammation can spread throughout the circulatory system, leading to systemic inflammatory response syndrome, also known as a cytokine storm. This systemic inflammation can cause septic shock, where blood pressure drops dangerously low and organs can no longer be perfused, leading to multi-organ failure and death.
Complications
SARS-CoV-2 can cause a number of complications, including direct damage to organs that express ACE-2 receptors, such as the heart, gastrointestinal tract, kidneys and liver. In severe disease, blood clots can form in the lungs and elsewhere, which could be a result of the virus or the inflammatory response, or both.
Virus lifecycle and potential therapies
There are no medicines currently approved to treat or prevent COVID-19 and no randomised controlled trial evidence that any treatment beyond best supportive care improves outcomes. However, many new and repurposed medicines are being investigated as part of nationally prioritised clinical trials.
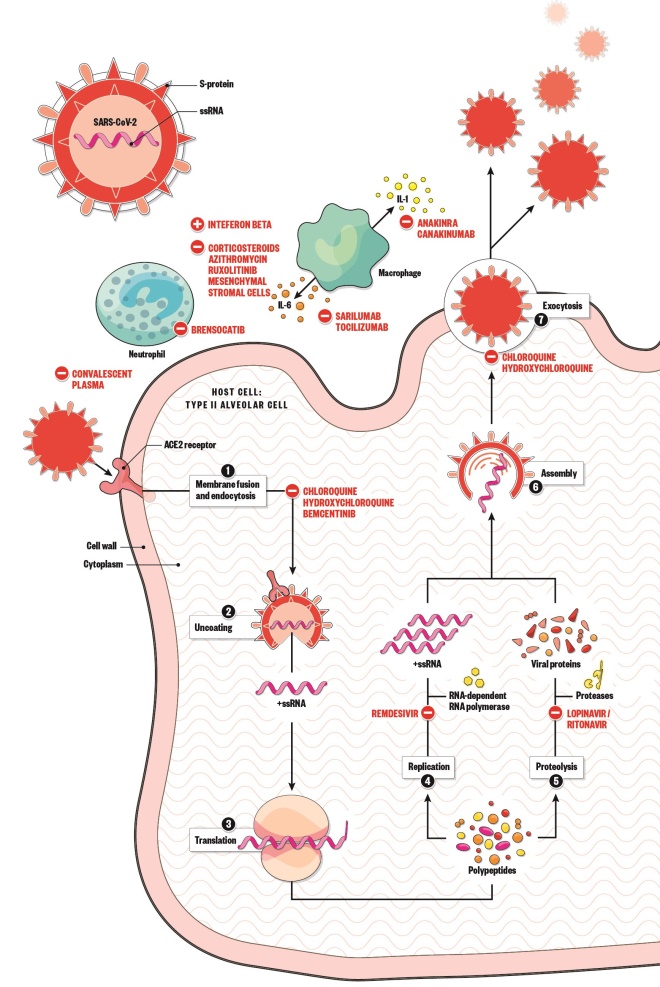
- Spike protein on the surface of SARS-CoV-2 uses the ACE2 receptor to gain entry to the host cell.
- The virus releases its RNA.
- RNA is translated into polypeptides.
- Some polypeptides form RNA-dependent RNA polymerase, which is needed to make more RNA.
- Other polypeptides are cleaved by proteases to produce viral proteins.
- Proteins and RNA are assembled into a new virion.
- New virion is released from the host cell.
Antivirals
Bemcentinib: selectively inhibits AXL kinase activity, which blocks viral entry and enhances the antiviral type I interferon response. (UK prioritised trials: ACCORD-2)
Chloroquine and hydroxychloroquine: block viral entry by inhibiting glycosylation of the ACE2 receptor, increasing endosomal pH (which also affects virion release), and blocking release of the viral genome. They also have immunomodulatory effects. (PRINCIPLE, RECOVERY and REMAP-CAP [hydroxychloroquine], COPCOV [chloroquine])
Lopinavir (boosted with ritonavir): inhibits proteases and, therefore, could inhibit 3-chymotrypsin-like protease, which plays a crucial role in viral replication of SARS-CoV-2. (RECOVERY, REMAP-CAP)
Remdesivir: inhibits RNA-dependent RNA polymerase, and therefore interferes with RNA replication. (ACTT, GS-5774, GS-5773)
Immune modulators
Anakinra and canakinumab:
inhibit IL 1, which is vital in the immune response to SARS-CoV-2. (REMAP-CAP [anakinra], CAN-COVID [canakinumab])
Azithromycin: has immunomodulatory properties that have shown benefit in inflammatory lung diseases. (PRINCIPLE, RECOVERY)
Brensocatib: reversibly inhibits dipeptidyl peptidase 1 — an enzyme that catalyses the activation of neutrophil serine proteases — which may help to reduce progression of lung injury. (STOP-COVID )
Convalescent plasma: antibodies bind and neutralise the virus as well as activating other immune cells to respond to the infection. (REMAP-CAP)
Corticosteroids: reduce the host’s inflammatory response to SARS-CoV-2 in the lungs. (RECOVERY)
Interferon beta: induces an initial innate antiviral response across a wide range of cell types and later mediates the adaptive immune response. (REMAP-CAP, SARS-CoV-2 infection)
Ruxolitinib:
modulates the immune response by regulating overactive signalling through the janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway. (RUXCOVID)
Mesenchymal stromal cells: secrete anti-inflammatory and immunomodulatory cytokines, produce pro-epithelial and endothelial repair factors and have antimicrobial properties, all of which may aid virus clearance, reduce injury and promote tissue repair in ARDS. (REALIST )
Sarilumab and tocilizumab: inhibit IL-6, which is vital in the immune response to SARS-CoV-2. (RECOVERY, a study to evaluate TCZ in patients with severe COVID-19 pneumonia [tocilizumab], REMAP-CAP [both])
Key
ACE-2: angiotensin converting enzyme-2
CO2: carbon dioxide
IL-1: interleukin 1
IL-6: interleukin 6
O2: oxygen
ROS: reactive oxygen species
s-protein: spike protein
ssRNA: single-stranded RNA
TNF-α: tumor necrosis factor alpha
References
Sources: National Institute for Health Research; JAMA 2020. doi: 10.1001/jama.2020.6019; National Institutes of Health; NHS England and NHS Improvement. Cell Death & Differentiation 2020; 27:1451.
Editorial advisers: Stephen Hughes, consultant antimicrobial pharmacist, Chelsea and Westminster NHS Foundation Trust. Ian Hall, director of the NIHR’s Nottingham Biomedical Research Centre. Illustrations: Alisdair MacDonald