Shutterstock.com
Open access article
The Royal Pharmaceutical Society has made this article free to access in order to help healthcare professionals stay informed about an issue of national importance.
To learn more about coronavirus, please visit: https://www.rpharms.com/resources/pharmacy-guides/wuhan-novel-coronavirus

Source: Shutterstock.com
Mass antibody testing forms pillar 3 of the government’s COVID-19 testing strategy
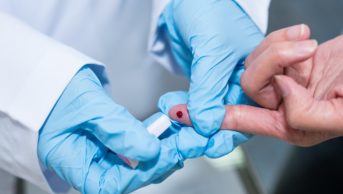
The UK government has begun recruiting volunteers to evaluate COVID-19 antibody tests that use finger-prick blood, after community pharmacies were advised to stop providing such tests.
In a statement to The Pharmaceutical Journal, the Department of Health and Social Care (DHSC) said it was working with Public Health England (PHE) and the Medicines and Healthcare products Regulatory Agency (MHRA) to recruit 2,500 volunteers from across England for the study, which will look at “the potential of home-based antibody tests” using finger-prick blood.
Mass antibody testing forms pillar 3 of the government’s COVID-19 testing strategy, published on 4 April 2020, which promised “to further develop any promising [antibody] tests for clinical and then potential home use”.
The DHSC confirmed the study is separate to the ongoing programme of research led by Imperial College London and Ipsos Mori around antibody testing using finger-prick blood.
The government-led study, which the DHSC said began recruiting in early June 2020 and is expected to produce initial results by late-summer 2020, comes after the MHRA asked “high street pharmacies and private healthcare providers to temporarily stop selling certain antibody tests on 29 May 2020.
The MHRA said the laboratory-based tests — manufactured by Roche and Abbott — had been validated using venous blood drawn by a healthcare professional but were on sale to the public on the basis that finger-prick blood, drawn at home, could be used instead.
This raised questions around the reliability of the tests, with Superdrug temporarily suspending the sale of the Abbott antibody test from its website.
The MHRA added that resolving regulatory and safety concerns would involve “further validation of the sample collection kits and the sample type and ensuring that result and supporting information is helpful and accurate”.
PHE had also advised against the use of “rapid point-of-care tests” for COVID-19 antibodies — which deliver a result in ten minutes — in community pharmacies or at home, noting that there is “little information on the accuracy” of these tests.
However, in a statement issued to The Pharmaceutical Journal on 22 June 2020, a DHSC spokesperson said the body was now recruiting “volunteers from the NHS and wider public service for a study exploring the potential of home-based antibody tests,” including those using finger-prick blood.
“No reliable home test has yet been found, and we do not know whether antibodies indicate immunity from reinfection or transmission,” they said, adding that the research “is part of our ongoing surveillance work to increase our understanding of how to tackle this virus”.
The DHSC added that volunteers will be drawn from individuals who have tested both positive and negative for the virus previously, as well as those who have not been tested before.
It said the volunteers would be evaluating how effective these finger-prick tests are when used by the public, and how easy they are to use.
The volunteers are also expected to help develop other kinds of tests that could indicate past coronavirus infections.
PHE would not provide further details on the type of finger-prick antibody tests that will be evaluated
However, manufacturers of such tests have already expressed interest in selling their products through community pharmacies.
David Campbell, director of SureScreen Diagnostics manufacturing company, said its test had received a CE mark for use by healthcare professionals and was “working extremely well in the independent validations conducted in the UK”.
“Our main customers are hospitals, GPs and occupational health,” he said, adding: “Pharmacies could be a great place for testing to be conducted, I am sure [PHE and the government] are considering this.”
Brigette Bard, chief executive at diagnostics manufacturer BioSURE, told The Pharmaceutical Journal that its antibody test was similar to its HIV test that is already on the market.
“Our plan is to ensure the BioSURE COVID-19 antibody self-test will be available through our usual consumer channels, which would include direct sales on our website, and community and high street pharmacies,” she said.
“We are currently awaiting regulatory approval; however, once we receive this, we are set for mass production,” she added.
The MHRA confirmed to The Pharmaceutical Journal that there is “no regulatory reason” finger-prick antibody tests, used within their CE-marked scope, cannot be put on the market in the UK, but pointed to the PHE advice advising against their use.