Key points:
- Electronic prescribing (EP) in the hospital setting has the potential to improve safety through reduction of errors and adverse drug events.
- Evidence for the effects of EP on workflow and timesaving is mixed.
- Unintended consequences of the computerisation of prescribing are well documented.
- There are a lack of UK-specific data and the heterogeneity of existing international studies makes it difficult to extrapolate evidence to the UK.
- There is great potential for digitised health in the UK, including the interoperability of healthcare and increasing patients’ involvement with their medicines.
Introduction

Courtsey of Airedale NHS Foundation Trust
Hospital electronic prescribing (EP) is an important item on the UK government health policy agenda, with hospitals in England expected to be paperless by 2020. It has been argued that the greatest benefits are associated with integrated EP systems because they offer potential improvements in communication among patients, prescribers, pharmacists and other stakeholders involved in medicines management. In the image, healthcare professionals using an EP system on a ward round
Government policy is increasingly promoting the use of technology in healthcare. For example, the UK government has launched several initiatives and funding streams to drive technology use within the NHS[1],
[2]
. Similarly, both the US government and the European Commission have recognised the potential for healthcare information technology (HIT) and encouraged its meaningful use[3],
[4]
. Despite worldwide advances in HIT, implementation of hospital electronic prescribing (EP) is lagging behind in many countries. In the UK, although the use of EP is ubiquitous in primary care[5],
[6]
, deployment in secondary care remains slow and patchy[6],
[7]
. Of 101 English acute trusts responding to a national survey of EP systems, more than two thirds (70 trusts; 69%) had at least one form of EP in use at the time of the survey, with over half of these having more than one system (39 trusts; 56%)[7]
. However, EP was often used in limited clinical areas and for limited types of prescribing. This may be because processes and the level of care provided are far more complex in secondary care[6]
. The low uptake is comparable to many other European countries. A study of the use of EP in seven different countries, including the UK, reported that despite the variations in healthcare systems, both the uptake of EP and the extent of systems’ interoperability were low in all seven locations[8]
. Hospital-wide EP was reported to be least widely used in Germany, France and the UK[8]
.
While there is no universally agreed definition for EP, definitions usually refer to the ordering or prescribing of medication electronically. For instance, the US eHealth initiative defined EP as “the use of computing devices to enter, modify, review, and output or communicate, drug prescriptions”[9]
. NHS Connecting for Health defined EP as “the utilisation of electronic systems to facilitate and enhance the communication of a prescription or medicine order, aiding the choice, administration and supply of a medicine through knowledge and decision support and providing a robust audit trail for the entire medicines use process”[10]
. In the United States, the term ‘computerised provider (or physician, or prescriber) order entry (CPOE)’ tends to be used instead of ‘EP’ in the hospital setting, where the scope of CPOE may include other types of medical orders such as laboratory tests and radiology[11]
.
Hospital EP systems are therefore far more complex than those used in primary care settings. They can include some, or all, of a range of different functions, and may be implemented in a wide range of organisational contexts and involve different healthcare professionals at different points of care. A report published in 2009 suggested that the UK hospital EP software market comprised four major types of systems[5]
:
- Pharmacy-based systems (systems emanating from providers of pharmacy stock control solutions);
- Clinical specialty-based systems (e.g. cancer systems, renal medicine and intensive care systems);
- Components or modules of larger hospital information systems (such as part of an integrated electronic health records solution);
- Home-grown software (systems developed locally by in-house informatics teams).
In 2014, a specific classification of commercial EP systems used in UK hospitals was proposed[12]
. Two broad categories of commercial systems were identified: bespoke systems and packaged applications. Bespoke systems were defined as systems designed to meet particular needs of a single organisation, while packaged applications were standard systems designed to meet requirements of different hospitals, which may then be configured to meet certain requirements of a particular hospital. The authors further divided packaged applications into four subcategories: standalone systems, modules within an integrated hospital information system, speciality systems, and functionalities spread over several modules[12]
.
EP and CPOE systems vary in functionalities, both those that are possible within the system itself and those that are configured for use in a given organisation. Therefore, EP can range from simple systems that allow basic prescribing to advanced systems that are integrated with clinical (computerised) decision support systems (CDSS) of varying sophistication[13]
. It has been argued that the greatest benefits are associated with integrated EP systems because they offer potential improvements in communication among patients, prescribers, pharmacists and other stakeholders involved in medicines management[14]
. Hospital EP is an important item on the UK government health policy agenda. English hospitals are expected to be paperless by 2020[15]
and therefore the deployment of EP systems is expected to rise rapidly in the near future.
This review aims to summarise the available evidence on the impact of EP on patient safety in the inpatient setting, with a focus on implications for the UK. The evidence for the effects of EP on medication errors, adverse drug events (ADEs), workflow, and healthcare professional communication is also discussed. Potential unintended consequences of EP are highlighted, together with how they can be identified and mitigated. The article concludes with considerations of the evolution of EP in hospitals, especially in relation to advances in HIT and interoperability, and how these developments may be used to benefit patient safety.
Sources and selection criteria
The authors aimed to identify systematic and narrative reviews, including reviews of reviews, which examined the effects of EP on the safety of the medicines management process in the inpatient setting. A literature search was conducted using PubMed/MEDLINE and EMBASE for articles published between January 2000 and December 2015. The search terms used were ‘electronic prescribing’, or ‘CPOE’, or ‘computerised provider order entry’, or ‘computerized provider order entry’. For inclusion, reviews had to be published in English and focus on EP or CPOE use in the hospital inpatient setting. Reviews focused on settings other than inpatients and those limited to a specific patient population, such as paediatrics or critical care patients, were excluded. Reviews that examined evidence for the effects of EP on the following outcomes were included: medication errors, ADEs, workflow, healthcare professional communication, and unintended consequences of EP systems use. Inclusion and exclusion criteria are detailed in ‘Table 1: Inclusion and exclusion criteria’.
| Criterion | Included | Excluded |
|---|---|---|
| EP: electronic prescribing, CPOE: computerised provider order entry, CDSS: clinical decision support system, ADE: adverse drug event | ||
| Sources | MEDLINE, EMBASE | Other databases, grey literature |
| Dates | 2000–2015 | Reviews published before, or after, this period |
| Review type | Narrative reviews, systematic reviews, reviews of reviews | Other types of publication and publications where full text could not be obtained |
| Language | English | Other languages |
| Intervention | EP or CPOE with, or without, CDSS | Other interventions |
| Outcome measure | Medication errors, ADEs, workflow, and healthcare professional communication | Other outcome measures |
| Setting | Hospital inpatient setting | Primary care and ambulatory care settings |
| Population | General inpatient populations | Reviews limited to specific patient populations (e.g. paediatrics only or critical care patients only) |
Discussion
The search yielded 879 potential publications, of which 103 were duplicates. Initial review of title and abstract resulted in 41 publications for full text review, of which 31 were subsequently excluded (see figure 1 for a depiction of the screening and selection process).
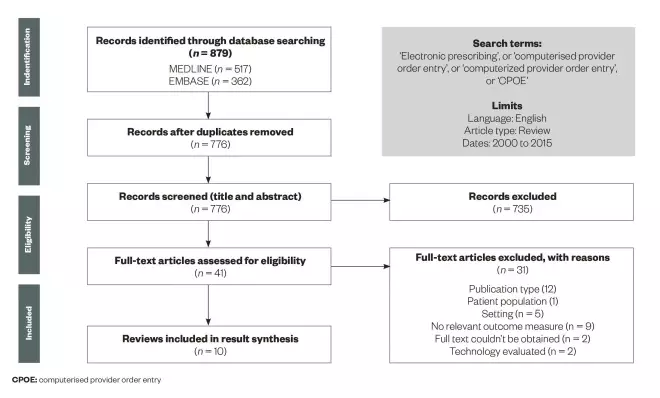
Screening and selection process for published articles on electronic prescribing
Source: Clinical Pharmacist
Articles had to be published between January 2000 and December 2015. For inclusion, reviews had to be published in English and focus on EP or CPOE use in the hospital inpatient setting
Of the ten reviews that were included[16],
[17],
[18],
[19],
[20],
[21],
[22],
[23],
[24],
[25]
, nine evaluated the effects on error and/or ADE rates[16],
[17],
[18],
[19],
[20],
[21],
[22],
[24],
[25]
, two reported effects on workflow[22],
[23]
, and one reported unintended consequences of systems use[22]
. The majority of the original studies included in the reviews identified were from the United States; only a few original studies were conducted in the UK. Details of all ten included reviews are summarised in ‘Table 2: Summary of reviews that examined the evidence for the effects of EP/CPOE with, or without, CDSS on the safety of the medicines management process’.
| Review (year) | Outcome measures | Aim | Included studies (number, settings, countries) | Summary of findings |
|---|---|---|---|---|
| EP: electronic prescribing, CPOE: computerised provider order entry, CDSS: clinical decision support system, ADE: adverse drug event, ICU: intensive care unit * We were unable to report location for studies cited in reviews of reviews, or where reviews comprised large numbers of studies | ||||
| Kaushal et al. (2003)[20] | Medication errors, ADEs, prescribing behaviours | A systematic review to assess the effect of CPOE (and CDSS) on medication error rates and ADEs | Total number of studies: 5 studies of CPOE systems (plus others of CDSS alone) Location: All in the United States | Two studies demonstrated a decrease in serious medication error rates, one showed improvement in corollary orders, one showed improvement in five prescribing behaviours, and one demonstrated improvement in nephrotoxic drug dose and frequency. |
| Rothschild (2004) [19] | Medication errors, ADEs | A narrative review to evaluate the effects of CPOE on clinical and surrogate outcomes in hospitalised patients in both general and ICU settings | Total number of studies: 7 of 18 studies evaluated the effects of CPOE on medication prescribing Location: All in the United States | Three of the seven studies demonstrated a significant reduction in serious medication errors, including ADEs while one study failed to show a reduction in ADEs. Two of the seven studies demonstrated reduced patient length of stay. Surrogate outcome improvements associated with CPOE were evident in two of the seven studies. |
| Wolfstadt et al. (2008)[16] | ADEs | A systematic review to assess the effects of CPOE with CDSS on ADEs | Total number of studies: 10 studies Location: Belgium (1), United States (9) | Five studies showed a significant decrease in ADEs. Four studies reported a non-significant reduction in ADE rates, and one study showed no effect. |
| Ammenwerth et al. (2008)[17] | Medication errors, ADEs, potential ADEs | A systematic review analysing the relative risk reduction on medication error rates and ADEs by CPOE | Total number of studies: 27 studies (15 medication errors, 2 ADEs and 10 evaluating both) Location: United States (17), Belgium (1), Canada (2), Australia (1), Singapore (1), Japan (1), France (1), UK (2), Israel (1) | Of 25 studies, 23 analysed the effects on the medication error rate showed a significant relative risk reduction of 13% to 99%. Six of the nine studies that analysed the effects on potential ADEs showed a significant relative risk reduction of 35% to 98%. Four of the seven studies that analysed the effect on ADEs showed a significant relative risk reduction of 30% to 84%. |
| Eslami et al. (2008)[25] | Adherence to guidelines, safety, time, ADEs, cost and cost effectiveness | A systematic review to assess the impact of CPOE in hospitalised patients | Total number of studies: 67 studies (22 adherence, 7 alerts and appropriateness of alerts, 21 safety, 7 time, 23 costs and (organisational) efficiency, 10 satisfaction, usage and usability) Location: United States (56), European Union (7), and across Brazil, Canada and Australia (4 in total) | Adherence to guideline or to computerised recommendation increased, prescribing errors decreased, although there were some recent negative studies, there was no evidence on the effect of CPOE systems on ADEs. Studies on cost and effectiveness showed mixed results and studies on alerts showed mixed results. Direct order entry time increased. When indirect time is also measured, the overall time did not change, or even decreased. |
| Reckmann et al. (2009)[18] | Prescribing errors | A systematic review to examine effects of CPOE systems on reducing prescribing errors among hospital inpatients | Total number of studies: 13 papers reporting 12 studies Location: United States (6), UK (3), Europe (2), Israel (1) | Nine studies demonstrated a significant reduction in prescribing error rates for all or some drug types. Several studies reported increases in the rate of duplicate orders and failures to discontinue drugs, often attributed to inappropriate selection from a dropdown menu or to an inability to view all active medication orders concurrently. |
| Niazkhani et al. (2009)[23] | Clinical workflow | A review to understand the impact of CPOE systems on clinical workflow | Total number of studies: 51 studies Location: United States (38), Japan (1), Norway (1), UK (1), Canada (1), France (3), Denmark (1), Australia (3), unknown (2) | Studies showed mixed effects of CPOE on workflow. Most reported positive outcomes: legible orders, remote accessibility of the systems, and the shorter order turnaround times. Most reported negative outcomes: time-consuming and problematic user-system interactions, and the enforcement of a predefined relationship between clinical tasks and between providers. |
| Radley et al. (2013)[21] | Medication errors | A systematic review to estimate medication error reduction in hospitals attributable to EP through CPOE | Total number of studies: 9 studies Location: All in the United States | Eight of nine studies showed a decrease in medication error frequency after CPOE implementation, while one study reported an increase (23%) in medication errors. |
| Ranji et al. (2014)[22] | Prescribing errors, ADEs, workflows, unintended consequences | A narrative review of systematic and narrative reviews to assess the evidence regarding the effectiveness of CPOE with CDSS at preventing clinically significant ADEs | Total number of studies: 5 systematic reviews, 1 narrative review, 2 controlled trials Location: Multiple* | CPOE with CDSS was consistently reported to reduce prescribing errors, but did not appear to prevent clinical ADEs in either the inpatient or outpatient setting. Implementation of CPOE with CDSS profoundly changed staff workflow, and often led to unintended consequences and new safety issues (such as alert fatigue), which limited the system’s safety effects. |
| Nuckols et al. (2014)[24] | Errors, ADEs | A systematic review to assess the effectiveness of CPOE at reducing preventable ADEs and errors in hospital-related settings | Total number of studies: 16 studies (10 evaluated effects on errors, 6 evaluated effects on errors and preventable ADEs) Location: United States (8), UK (2), Pakistan (1), Netherlands (1), Australia (1), Belgium (1), Israel (1), Spain (1) | CPOE was associated with half as many preventable ADEs (pooled risk ratio [RR] = 0.47, 95% confidence interval [CI] 0.31–0.71) and medication errors (RR = 0.46, 95% CI 0.35–0.60). |
Medication errors and ADEs
Nine reviews examined the effects on error rates and/or ADEs and suggest that inpatient EP use is associated with benefits in reduced medication errors and, to a lesser extent, ADEs. However, the evidence is limited by the reviews’ varied inclusion and exclusion criteria, variation in definitions of errors and ADEs, relatively weak study designs of the included studies, and a lack of contextual data about the systems implemented. Included studies were also heterogeneous in design, setting and methods of evaluation. Moreover, most systematic reviews were based on a preponderance of US studies, with only four UK studies reported in six papers[26],
[27],
[28],
[29],
[30],
[31]
(see ‘Table 3: Characteristics of the studies that evaluated the introduction of EP, in comparison with paper medication orders, in UK hospitals’).
| Study (year) | Time of study | Hospital | Clinical setting | Patient population | System evaluated | System type | Functionality | Study design | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|
| CDSS: clinical decision support system | |||||||||
| Evans et al. (1998)[26] | 1996 | The John Radcliffe Hospital, Oxford | Intensive care unit | Critical care patients | Hewlett Packard CareVue patient information system | Commercial | No CDSS | Before and after study | Accuracy, completeness of medication orders, time |
| Mitchell et al. (2004)[27] | 2002 | Southmead Hospital, Bristol | General surgery | No restriction | Clinical Manager 3.0A, iSoft UK PLC | Commercial | No CDSS | Before and after study | Medication error rates |
| Shulman et al. (2005)[28] | 2001–2002 | University College Hospitals, London | Intensive care unit | Critical care patients | QS 5.6 Clinical Information System, GE Healthcare | Commercial | No CDSS | Before and after study | Medication error rates |
| Franklin et al. (2007)[29], [30], [31] | 2003–2004 | London teaching hospital | General surgery | No restriction | ServeRx V.1:13, MDG Medical | Commercial | No CDSS | Before and after study | Prescribing error rates, medication administration error rates, confirmation of patient identity, staff time |
All four UK research studies identified in the reviews evaluated pilot introductions of EP systems in specific clinical areas, and revealed mixed findings in relation to the effect on medication errors. All used uncontrolled before and after study designs. Evans et al. conducted a study in an intensive care unit (ICU) to compare the accuracy, completeness and time requirements of EP versus handwritten prescriptions[26]
. They found that although electronic prescriptions were more complete (100% vs. 47% respectively), errors in prescribing were not reduced by EP. The percentages of correct medication orders for intravenous fluids, intravenous infusions and intermittent IV drugs were 64%, 47.5% and 90% for handwritten prescriptions, compared with 48%, 32% and 90% for electronic charts, respectively.
Mitchell et al. evaluated a pilot EP and electronic medication administration record (eMAR) system in surgical wards, operating theatres and the recovery ward of a teaching hospital[27]
. A total of 4,927 computerised prescriptions were written during the pilot. An audit of handwritten medication administration records identified significant omissions of patient and drug information. Omissions were less frequent with eMAR, with all mandated fields being 100% complete. An audit of electronic drug orders identified 143 errors, giving an error rate of 2.9% of medication orders. The highest error rates were seen in the first week of the project (6.4%), half of which were caused by the selection of the wrong formulation of the required drug. The authors identified errors specific to the use of EP/eMAR in 1.2% of electronic orders (57 of 4,927).
Another UK study compared the impact of EP (without decision support) with handwritten prescribing on the frequency, type and outcome of medication errors in an ICU[28]
. The authors found that medication errors were significantly lower with EP (117 errors in 2,429 prescriptions; 4.8%) than with handwritten prescriptions (69 errors in 1,036 prescriptions, 6.7%; P <0.04). The prevalence of errors reduced with time following EP introduction (P <0.001).
Finally, in a study conducted in a surgical ward of a UK teaching hospital, researchers assessed the impact of a closed-loop EP and automated dispensing system on prescribing errors, administration errors and staff time[29],
[30],
[31]
. The system reduced prescribing errors (3.8% of medication orders pre-intervention and 2.0% after intervention, P <0.001), medication administration errors (7.0% pre-intervention and 4.3% after intervention, P =0.005), and increased confirmation of patient identity before administration.
Effect on workflow and healthcare professionals’ communications
Two reviews examined effects on workflow[22],
[23]
and one examined effects on time[25]
. One UK study reported effects on workflow[26]
and found that the mean time taken to prescribe one medication order was 20 seconds for medication orders and 55 seconds for EP[26]
. There were no data about communication with patients or their involvement in the medicines management process.
Findings of the identified reviews suggest that EP is associated with significant workflow changes but the effects of these changes are mixed. It is therefore hard to reach a definitive conclusion as to whether these changes result in an overall positive or negative effect on patient care. The reviews also indicate that although order entry times seem to be increased by computerisation, the overall time spent on care may be similar or reduced. This evidence is, however, extrapolated from international literature, predominantly US studies where medication-related workflow is different to the UK. Moreover, many studies looked at subsets of clinical workflow and not the overall workflow across a whole day. Another major limitation is the lack of studies looking at medicines-related workflows specifically, as US CPOE systems are generally used for ordering more than just medicines.
Unintended consequences of EP and how can they be mitigated
One review examined unintended consequences and new safety issues of EP use, and concluded that they limit the system’s safety benefits[22]
. However, there was no UK evidence included. The authors also highlighted that there is no standardised approach to avert alert fatigue[22]
.
Overall consequences of changing from paper to EP
International evidence shows that EP may improve the safety of inpatient medicines management processes, reduce medication errors and, to a lesser extent, reduce ADEs. However, unintended consequences, including new errors, may occur. Evidence on the effects of EP on workflow is limited as most studies evaluated CPOE systems in general and did not specifically explore medicine-related workflow. The application of this evidence to practice in the UK is difficult as most of the existing evidence is from the United States where prescribing and administration practices, and workflow, are very different to those in the UK[32]
. For instance, there are differences in medication documentation, labelling and supply practices, as well as variation in policies governing patients’ own medication use[32]
. Therefore, the benefits and negative consequences of changing from paper to EP may be different in the UK setting.
Implications for practice
There are potential patient safety benefits from EP use, but the realisation of these benefits is dependent on successful implementation and utilisation. EP adoption results in significant changes to existing practice[24]
. Healthcare representatives from all clinical areas should therefore be a part of the EP adoption and evaluation team to include all perspectives and ensure that the new system meets the hospital’s specific needs[5],
[33]
. Building the right teams and equipping staff with project management and IT skills are important for success[5]
.
As illustrated earlier, most studies in this area have been conducted in the United States. While robust UK studies are under way[34]
, it is important to capture the impact before, during and after a wide range of EP implementations wherever possible, to provide further UK evidence.
While there is much promotion of the benefits of EP, various unintended negative consequences of EP systems have been described in the literature[35],
[36],
[37]
. Clinical unintended adverse consequences resulting from CPOE implementation have been classified into nine types[38]
. These comprise: more or new work for clinicians, unfavourable workflow, ‘never-ending system demands’, problems related to paper persistence, untoward changes in communication patterns and practices, negative emotions, generation of new kinds of errors, unexpected changes in the power structure, and overdependence on the technology[38]
.
New medication errors can occur when prescribers pick from a drug list (drop-down menu) or while filling free-text fields in an electronic prescription. This could potentially be minimised by reducing the size of drop-down lists, minimising free-text prescribing, and building well-designed, pre-defined ‘order sentences’ and ‘care sets’ into the system. Order sentences are defined as ‘a complete pre-written medication order that includes dose, dose form (when necessary), route of administration, frequency and, if appropriate, a PRN (pro re nata) flag and reason’[39]
. Care sets allow clinicians to launch a combination of related orders (e.g. medication, laboratory tests and/or other examinations) for a specific clinical situation.
Another consequence of CPOE that may contribute to errors is overriding alerts, especially those warning of severe drug interactions or significant allergies. Alert fatigue is well documented in the literature[40],
[41],
[42]
. There is no standardised approach to avert alert fatigue[22]
, although tailoring alerts has been suggested as a solution[43],
[44]
. The integration of patient, illness and medicine information, as well as the development of an alert hierarchy to generate, at most, one clinically relevant alert per prescription, have been suggested as measures to reduce alert fatigue[45]
.
Future opportunities for digitalised health in the UK
Integration and interoperability of healthcare
Widespread use of EP in hospitals, particularly in the context of integrated systems, offers an opportunity to improve patient safety and quality of care. First, the rise of integrated approaches to the delivery of healthcare, and HIT interoperability, may enhance care coordination through improved access and exchange of clinical data[46]
. Increased access to medication-related information by healthcare professionals and patients, as well as increased access to clinical data at the point of prescribing, would be expected to improve efficiency and reduce clinical risks[47]
. Interoperability may also offer an opportunity for improved exchange of clinical data between primary and secondary care. Second, there is potential for healthcare providers and patients to assume different roles in the delivery of healthcare. Connectivity could lead to time savings[47]
and thus provide healthcare professionals with an opportunity to focus on more clinical roles. Moreover, integration and interoperability supports team-based care approaches. Similar to healthcare providers, patients will be able to access and communicate information with their caregivers efficiently, which may support patient involvement in their own safety[48]
. Finally, interoperability may help monitor policies both locally and nationally, and facilitate secondary use of data for audit and feedback, which may contribute to improved patient care[49]
.
Inpatients’ involvement with their medication in the context of EP
It is increasingly recognised that it is essential to involve patients with their medication[50],
[51]
. Recognition has also been given to the importance of patient involvement in enhancing safety[51],
[52]
. Such patient involvement can increase satisfaction, improve health outcomes and reduce the likelihood of avoidable harm[53]
. Patient safety activities relating to inpatient medication include — but are not limited to — viewing their inpatient medication records, prompting staff to avoid dose omissions, providing information to aid handover between shifts and professional groups, and raising queries with prescribers, pharmacists or nursing staff.
While EP has the potential to facilitate patient and carer involvement with inpatient medication — for example, by the creation of patient-friendly interfaces — research in this area suggests that it currently acts as a barrier[54],
[55],
[56]
. Few EP systems include a patient interface[55]
and qualitative studies[54],
[56]
suggest that patients have less access to their electronic medication records because a healthcare professional login is required. In addition, where electronic records are not available at a patient’s bedside, conversations about the patient’s medication appear to be less likely to happen in front of them and healthcare professionals may be unable to answer their queries[54],
[56]
.
Limitations of this review
The objective of this review is to provide a broad overview, and therefore, a simple search strategy was employed. Some relevant reviews and individual UK studies may have been missed because of limitations in the search strategy; for instance, the use of other search terms may have yielded more results. Papers where the full text was not obtainable or not written in English were excluded and a search of the grey literature was not conducted. The search strategy was conducted by one researcher only and the quality of the included reviews was not assessed.
Conclusions
Safety in hospitals could be improved by EP use. However, the extent to which the existing evidence is applicable to UK settings is not yet clear. Moreover, benefits are likely to be dependent on how systems are implemented and used in practice. Further evaluations of the effects of EP systems on the safety of the medicines management process in UK hospitals are required to inform both policymakers and end users. There may be great potential to improve patient safety, and quality of care, through greater integration and interoperability of HIT in the UK. This could be achieved through improved access and exchange of clinical data, and supporting a team-based care approach. EP should ideally be used to facilitate patient and carer involvement with inpatient medication, rather than acting as a barrier.
Zamzam Ahmed is a PhD candidate at the Centre for Medication Safety and Service Quality, Pharmacy Department, Imperial College Healthcare NHS Trust & UCL School of Pharmacy, London. Sara Garfield is a research pharmacist at the Centre for Medication Safety and Service Quality, Pharmacy Department, Imperial College Healthcare NHS Trust & UCL School of Pharmacy, London. Yogini Jani is medication safety officer at University College London Hospitals NHS Foundation Trust, honorary lecturer at UCL School of Pharmacy and associate director at Medicines Use & Safety division, NHS Specialist Pharmacy Service. Seetal Jheeta is a research pharmacist at the Centre for Medication Safety and Service Quality, Pharmacy Department, Imperial College Healthcare NHS Trust , London. Bryony Dean Franklin is director and professor of medication safety at the Centre for Medication Safety and Service Quality, Pharmacy Department, Imperial College Healthcare NHS Trust & UCL School of Pharmacy, London. Correspondence to:
zamzam.ahmed.11@ucl.ac.uk
Financial and conflicts of interest disclosure:
Zamzam Ahmed is funded by the UCL School of Pharmacy Oversees Research Award (SOPORA), UCL School of Pharmacy. The Centre for Medication Safety and Service Quality is affiliated with the National Institute for Health Research (NIHR) Imperial Patient Safety Translational Research Centre. Bryony Dean Franklin is affiliated with the NIHR Health Protection Research Unit in Healthcare Associated Infection and Antimicrobial Resistance at Imperial College London in partnership with Public Health England. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health, or Public Health England. The funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The researchers are independent from the funders. Cerner are also part-funding a PhD studentship at UCL School of Pharmacy. The other authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilised in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Department Of Health. The NHS plan: a plan for investment, a plan for reform. 2000. Available at: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_118522.pdf (accessed April 2016).
[2] NHS England. Safer hospitals, safer wards: achieving an integrated digital care record. 2013. Available at: http://www.england.nhs.uk/wp-content/uploads/2013/07/safer-hosp-safer-wards.pdf (accessed April 2016).
[3] The Centers For Medicare And Medicaid Services. Medicare and Medicaid EHR Incentive Program Basics. 2013. Available at: http://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Basics.html (accessed April 2016).
[4] European Commission. eHealth Action Plan 2012–2020: Innovative healthcare for the 21st century. 2015. Available at: https://ec.europa.eu/digital-agenda/en/news/ehealth-action-plan-2012-2020-innovative-healthcare-21st-century (accessed April 2016).
[5] Cornford TSI, Jani Y, Barber N et al. Electronic prescribing in hospitals – challenges and lessons learned. Report for NHS Connecting for Health. 2009. Available at: http://webarchive.nationalarchives.gov.uk/20130502102046/http://www.connectingforhealth.nhs.uk/systemsandservices/eprescribing/challenges/Final_report.pdf (accessed April 2016).
[6] Car JBA, Anandan C, Cresswell K et al. The Impact of eHealth on the Quality and Safety of Healthcare: A Systematic Overview and Synthesis of the Literature. Report for the NHS Connecting for Health Evaluation Programme. 2008. Available at: https://www1.imperial.ac.uk/resources/32956FFC-BD76-47B7-94D2-FFAC56979B74/ (accessed April 2016).
[7] Ahmed Z, McLeod MC, Barber N et al. The Use and Functionality of Electronic Prescribing Systems in English Acute NHS Trusts: A Cross-Sectional Survey. PLoS ONE 2013;8(11):e80378. doi: 10.1371/journal.pone.0080378
[8] Aarts J & Koppel R. Implementation of computerized physician order entry in seven countries. Health Aff 2009;28(2):404–414. doi: 10.1377/hlthaff.28.2.404
[9] EHealth Initiative 2004. Electronic Prescribing: Toward Maximum Value and Rapid Adoption. Recommendations for Optimal Design and Implementation to Improve Care, Increase Efficiency and Reduce Costs in Ambulatory Care. Available at: http://c.ymcdn.com/sites/www.azhec.org/resource/resmgr/files/erx_toward_maximum_value_and.pdf (accessed April 2016).
[10] Connecting For Health. Challenges and lessons learned. 2012. Available at: www.connectingforhealth.nhs.uk/systemsandservices/eprescribing (accessed April 2016).
[11] Doolan DF & Bates DW. Computerized Physician Order Entry systems in hospitals: mandates and incentives. Health Aff 2002;21:180–188. doi: 10.1377/hlthaff.21.4.180
[12] Mozaffar H, Williams R, Cresswell K et al. Product diversity and spectrum of choice in hospital ePrescribing Systems in England. PLoS One 2014;9:e92516. doi: 10.1371/journal.pone.0092516
[13] Kuperman G, Bobb A, Payne TH et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc 2007;14(1):29–40. doi: 10.1197/jamia.M2170
[14] Dobrev AS, Stroetmann KA, Stroetmann VN et al. The conceptual framework of interoperable electronic health record and ePrescribing systems. 2008. Available at: http://www.ehr-impact.eu/downloads/documents/EHRI_D1_2_Conceptual_framework_v1_0.pdf (accessed April 2016).
[15] National Information Board. Personalised health and care 2020: using data and technology to transform outcomes for patients and citizens. A framework for action. 2014. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/384650/NIB_Report.pdf (accessed April 2016).
[16] Wolfstadt JI, Gurwitz JH, Field TS et al. The effect of computerized physician order entry with clinical decision support on the rates of adverse drug events: a systematic review. J Gen Intern Med 2008;23:451–458. doi: 10.1007/s11606-008-0504-5
[17] Ammenwerth E, Schnell-Inderst P, Machan C et al. The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J Am Med Inform Assoc 2008;15:585–600. doi: 10.1197/jamia.M2667
[18] Reckmann MH, Westbrook JI, Koh Y et al. Does computerized provider order entry reduce prescribing errors for hospital inpatients? A systematic review. J Am Med Inform Assoc 2009;16:613–623. doi: 10.1197/jamia.M3050
[19] Rothschild J. Computerized physician order entry in the critical care and general inpatient setting: a narrative review. J Crit Care 2004;19:271–278. doi: 10.1016/j.jcrc.2004.08.006
[20] Kaushal R, Shojania KG & Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med 2003;163(12):1409–1416. doi: 10.1001/archinte.163.12.1409
[21] Radley DC, Wasserman MR, Olsho LE et al. Reduction in medication errors in hospitals due to adoption of computerized provider order entry systems. J Am Med Inform Assoc 2013;20:470–476. doi: 10.1136/amiajnl-2012-001241
[22] Ranji SR, Rennke S & Wachter RM. Computerised provider order entry combined with clinical decision support systems to improve medication safety: a narrative review. BMJ Qual Saf 2014;23:773–780. doi: 10.1136/bmjqs-2013-002165
[23] Niazkhani Z, Pirnejad H, Berg M et al. The Impact of Computerized Provider Order Entry Systems on Inpatient Clinical Workflow: A Literature Review. J Am Med Inform Assoc 2009;16(4):539–549. doi: 10.1197/jamia.M2419
[24] Nuckols TK, Smith-Spangler C, Morton SC et al. The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev 2014;4(3):56. doi: 10.1186/2046-4053-3-56
[25] Eslami S, Keizer NF & Abu-Hanna A. The impact of computerized physician medication order entry in hospitalized patients: a systematic review. Int J Med Inform 2008;77:365–376. doi: 10.1016/j.ijmedinf.2007.10.001
[26] Evans KD, Benham SW & Garrard CS. A comparison of handwritten and computer-assisted prescriptions in an intensive care unit. Crit Care 1998;22:1–7. doi: 10.1186/cc129
[27] Mitchell D, Usher J, Gray S et al. Evaluation and audit of a pilot of electronic prescribing and drug administration. J Inform Tech Healthcare 2004;2(1):19–29.
[28] Shulman R, Singer M, Gildstone J et al. Medication errors: a prospective cohort study of hand-written and computerised physician order entry in the intensive care unit. Crit Care 2005;9(5):R516–R521. doi: 10.1186/cc3793
[29] Franklin BD, O’Grady K, Donyai P et al. The impact of a closed-loop electronic prescribing and administration system on prescribing errors, administration errors and staff time: a before-and after study. Qual Saf Health Care 2007;16:279–284. doi: 10.1136/qshc.2006.019497
[30] Donyai P, O’Grady K, Jacklin A et al. The effects of electronic prescribing on the quality of prescribing. Br J Clin Pharmacol 2007;65(2):230–237. doi: 10.1111/j.1365-2125.2007.02995.x
[31] Franklin BD, Birch S, Savage I et al. Methodological variability in detecting prescribing errors and consequences for the evaluation of interventions. Pharmacoepidemiol Drug Saf 2009;18(11):992–999. doi: 10.1002/pds.1811
[32] Brock TP & Franklin BD. Differences in pharmacy terminology and practice between the United Kingdom and the United States. Am J Health Syst Pharm 2007;64:1541–1546. doi: 10.2146/ajhp060444
[33] Cresswell KM, Slee A, Coleman J et al. Qualitative analysis of round-table discussions on the business case and procurement challenges for hospital electronic prescribing systems. PLoS One 2013;14:e79394. doi: 10.1371/journal.pone.0079394
[34] Schofield B, Cresswel K, Westbrook J et al. The impact of electronic prescribing systems on pharmacists’ time and workflow: protocol for a time-and-motion study in English NHS hospitals. BMJ Open 2015;5:e008785. doi: 10.1136/bmjopen-2015-008785
[35] Koppel R, Metelay JP, Cohen A et al. Role of computerized physician order entry systems in facilitating medication errors. J Am Med Inform Assoc 2005;293(10):1197–1203. doi: 10.1001/jama.293.10.1197
[36] Ash JS, Berg M & Coiera E. Some unintended consequences of information technology in health care the nature of patient care information system-related errors. J Am Med Inform Assoc 2004;11(2):104–112. doi: 10.1197/jamia.M1471
[37] Weiner JP, Kfuri T, Chan K et al. e-Iatrogenesis: The Most Critical Unintended Consequence of CPOE and other HIT. J Am Med Inform Assoc 2007;14:387–388. doi: 10.1197/jamia.M2338
[38] Campbell EM, Sitting DF, Ash JS et al. Types of unintended consequences related to computerized provider order entry. J Am Med Inform Assoc 2006;13:547–556. doi: 10.1197/jamia.M2042
[39] Kuperman G, Bobb A, Payne T et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc 2007;14(1):29–40. doi: 10.1197/jamia.M2170
[40] Lin CP, Payne TH, Nichol WP et al. Evaluating clinical decision support systems: monitoring CPOE order check override rates in the Department of Veterans Affairs’ Computerized Patient Record System. J Am Med Inform Assoc 2008;15:620–626. doi: 10.1197/jamia.M2453
[41] Payne TH, Nichol WP, Hoey P et al. Characteristics and override rates of order checks in a practitioner order entry system. Proc AMIA Symp 2002;602–606. PMID: 12463894
[42] Van Der Sijs H, Aarts J, Vulto A et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc 2006;13(2):138–147. doi: 10.1197/jamia.M1809
[43] Seidling HM, Schmitt SP, Bruckner T et al. Patient-specific electronic decision support reduces prescription of excessive doses. Qual Saf Health Care 2010;19:e15. doi: 10.1136/qshc.2009.033175
[44] Paterno MD, Maviglia SM, Gorman PN et al. Tiering drug-drug interaction alerts by severity increases compliance rates. J Am Med Inform Assoc 2009;16(1):40–46. doi: 10.1197/jamia.M2808
[45] Coleman JJ, Van Der Sijs H, Haefeli WE et al. On the alert: future priorities for alerts in clinical decision support for computerized physician order entry identified from a European workshop. BMC Med Inform Decis Mak 2013;13(1):111. doi: 10.1186/1472-6947-13-111
[46] Dixon BE, Vreeman DJ & Grannis SJ. The long road to semantic interoperability in support of public health: experiences from two states. Journal of Biomedical Informatics 2014;49:3–8. doi: 10.1016/j.jbi.2014.03.011
[47] Walker J, Pan E, Johnston D et al. The value of health care information exchange and interoperability. Health Aff (Millwood), Jan–Jun, Suppl web exclusives: W5-10-W5-8. 2005.
[48] Steichen O & Gregg W. Section Editors for the IMIA Yearbook Section on Patient-centered Care Coordination. Health Information Technology Coordination to Support Patient-centered Care Coordination. Yearbook of Medical Informatics, 2015;10(1):34–37. doi: 10.15265/IY-2015-027
[49] Paul MM, Greene CM, Newton-Dame R et al. The state of population health surveillance using electronic health records: a narrative review. Popul Health Manag 2015;18(3):209. doi: 10.1089/pop.2014.0093
[50] RPSGB and MSD. From compliance to concordance: achieving shared goals in medicine taking. London: RPSGB. 1997.
[51] Department of Health. Equity and Excellence: Liberating the NHS. London: Department of Health. 2010.
[52] Lawton and Armitage. The role of the patient in clinical safety. London: The Health Foundation. 2012.
[53] Coulter A & Ellins J.Patient focused interventions. A review of the evidence. London: The Picker Institute. 2006.
[54] Lee L, Robins R & Sheikh A. What does E prescribing mean for patients? A case study of the perspectives of hospital renal patients? Journal of Innovation in Health Informatics 2015;22(4):391–398. doi: 10.14236/jhi.v22i4.176
[55] Jheeta S, Lubrant C, Garfield S et al. Inpatient electronic prescribing – how involved are inpatients with their medication? International Journal of Pharmacy Practice 2015;23(S1):2–27.
[56] Garfield S,Jheeta S, Husson F et al. The role of hospital inpatients in supporting medication safety: a qualitative study. PLoS ONE 2016. doi: 10.1371/journal.pone.0153721
You may also be interested in

Reviewing prescribing practice in an adult critical care unit with a newly implemented electronic prescribing system
