Shutterstock.com
In the UK, around 4.5 million people have diabetes and an estimated 1.1 million people are living with diabetes but have not yet been diagnosed, which means that an estimated 6% of the UK population have the disease. Of those individuals who have been diagnosed, around 90% have type 2 diabetes and around 10% have type 1 diabetes[1]
.
The financial impact of diabetes on the UK NHS equates to around £10bn, 80% of which is spent on the complications of diabetes (see Box 1: Complications of diabetes), with foot ulceration considered to be one of the most serious complications[2]
. Foot ulceration is responsible for more hospital days than all other diabetes complications combined[3]
. The annual spend for diabetic foot ulceration and amputation is estimated to be just under £1bn, most of which is spent in primary, community and outpatient settings[4]
. Around £1 in every £140 of NHS spending in England is on footcare for people with diabetes[5],[6]
.
Box 1: Complications of diabetes
There are many short- and long-term complications that can result from diabetes. High blood sugars (hyperglycaemia) and the length of time that diabetes is present increase the risk of complications, which include:
- Damage to nerves (neuropathy);
- Damage to the eyes (retinopathy);
- Damage to the kidneys (nephropathy);
- Damage to the cardiovascular system.
There may also be an associated increased risk of infection, depression and Alzheimer’s disease.
Within the foot, complications include:
- Peripheral arterial disease (PAD);
- Neuropathy;
- Foot deformity;
- Charcot neuroarthropathy;
- Active foot problems.
Testing for these conditions forms part of the annual foot assessment that is part of a patient’s annual diabetic review, which is conducted by a suitably trained individual.
Sources: Nathan DM; DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: overview. Diabetes Care
2014;37(1):9–16. doi: 10.2337/dc13-2112; http://www.mayoclinic.org/diseases-conditions/diabetes/basics/complications/con-20033091
Diabetes is the most common cause of non-traumatic limb amputations, with 80% of cases being preceded by foot ulcers[6]
and, therefore, are considered preventable. The number of people with diabetes who, in any given week, have an active foot ulceration is estimated to be around 60,000–75,000 in England, and around 5–7% of people with diabetes will experience a foot ulcer at some point in their life[4]
. Mortality rates are high, with 50% of patients dying within five years of developing a diabetic foot ulcer, which rises to 70% if the patient has an amputation because this is believed to be associated with cardiovascular disease[7]
.
Owing to the high prevalence and financial burden of foot problems that are related to diabetes, in addition to the impact on patients’ quality of life, it is important that pharmacists and healthcare professionals are aware of symptoms and the relevant care pathways for these patients to ensure they receive the best care and education regarding how to manage their condition.
Foot complications
Peripheral arterial disease (PAD)
An atherosclerotic occlusive disease of the lower extremities, peripheral arterial disease (PAD) leads to inadequate blood supply to the lower limb, decreases peripheral tissue viability and increases the skin’s susceptibility to minor trauma, impairs wound healing and increases the risk of infection. If not caught early, it can result in necrosis or gangrene and loss of limb. The incidence of PAD in patients with diabetes is more severe and diffuse, often at a younger age, with calcification occurring more distally, and is related to an increased risk of mortality and impaired quality of life compared with people without diabetes[8]
.
It has been reported that PAD is a factor in a third of foot ulcers and is a significant risk factor in re-ulceration[9]
.
Information on vascular assessment of the foot is covered later.
Neuropathy
Peripheral neuropathy (PN) develops when nerves in the extremities are damaged. The exact aetiopathogenesis of PN is still not fully understood but recent evidence suggests that vascular dysfunction, with changes to the blood supply of the nerves, could be responsible[10]
.
PN is reported to affect 50% of patients with diabetes, and its prevalence increases with age and duration of diabetes[11]
. It is present in more than 50% of patients with type 2 diabetes aged over 60 years[12]
and in more than 40% of patients who have had type 1 diabetes for 20 years[13]
.
PN may be the most important pathological precursor of foot ulcers, with neuropathy reportedly present in 90% of patients with diabetes who also have a foot ulcer[14]
,[15]
.
The most common form of PN is distal symmetrical polyneuropathy. This involves the sensory, motor and autonomic nerve fibres, where the earliest changes tend to occur most distally (i.e. in the digits and progress proximally).
Sensory
neuropathy results in the loss of pain and thermal perception, and is usually asymptomatic. The patient is, therefore, unaware that they cannot appreciate the sensation of pain and, thus, are unaware that an injury has been sustained. As a result, foot pathologies such as corns, calluses, thickened nails and foot deformity (e.g. hallux abducto valgus [bunion]) all pose a risk. Without pain sensation, these pathologies may be left unnoticed (and therefore untreated), causing underlying tissue damage and ulceration.
Healthcare professionals will test a patient’s foot sensations as part of their diabetic annual foot assessment (see later section on assessing a diabetic foot at risk)[16]
. However, it is imperative to inform patients about the importance of wearing shoes that fit properly — sensory loss renders an individual unable to detect rubbing or trauma from an ill-fitting shoe. In fact, people with neuropathy may actually wear shoes that are too small, as they perceive them to be a good fit[17]
.
Diabetic peripheral neuropathic pain (DPNP) is estimated to affect between 16% and 26% of all people with diabetes. It is under-reported and undertreated, with 80% of patients experiencing moderate to severe pain[18]
. Patients complain of paraesthesia, shooting pains down the legs, lancinating pains, burning, hyperaesthesia, allodynia, metatarsalgia, and sensations of cold or warmth[19]
, more commonly at night. Its aetiology is unknown but there is some evidence that age, duration of diabetes, nephropathy, PAD and an increased waist circumference can be possible predictors for the development of painful neuropathy[20]
. The National Institute for Health and Care Excellence (NICE), England’s health technology assessment body, recommends duloxetine as first-line treatment for DPNP[21]
, with further recommendations for combination of drugs if duloxetine monotherapy fails. However, first-line treatment is not always possible because of polypharmacy, with patients already being on a number of medicines.
Motor neuropathy affects the nerves that control movement. This results in wasting of the intrinsic muscles of the foot. One study, using magnetic resonance imaging, found that small muscle wasting is common in a neuropathic foot, leading to a cavoid foot type[22]
. This typically presents as a high arched foot with clawed toes, causing increased pressure to be placed on metatarsal heads and apices of digits, which results in callus formation and, thus, further pressure distribution, disruption and risk.
Autonomic
neuropathy affects involuntary functions, such as thermo-regulation and sweat production. Damage to these nerves leads to lack of adequate moisture in the skin, which results in loss of elasticity, dry skin, callus and fissuring[23]
. It also results in arterio-venous shunting where oxygen bypasses capillary beds and sends oxygenated blood into the venous system, resulting in deprivation of oxygen and nutrients to tissues[24]
.
Assessment and identification of a diabetic foot at risk
A standard clinical examination will identify most patients at risk of foot ulceration. To assess a patient’s risk status of developing a foot ulcer, healthcare professionals look for the following: neuropathy, PAD, ulceration, callus, infection and/or inflammation, deformity, gangrene and Charcot arthropathy[7],[9]
. Patients with diabetes should receive an annual foot check-up as part of their care. However, Diabetes UK[25]
reports that only 36% of diabetic patients receive a full annual assessment and patients are not getting a good quality foot check or being advised of their risk status for developing foot ulcers[26]
. Assessment not only includes examination of the lower limbs and feet, but of the patient as a whole, including their history, foot pathology, and vascular and neurological assessment. Patients should be reminded to have their feet checked by a relevant healthcare professional at least once a year.
Taking the patient’s history
Long-term studies on people with type 1 and type 2 diabetes have shown that the longer the person has had diabetes or if they have poorly controlled diabetes, they are at an increased risk of complications, such as PAD and neuropathy. Therefore, duration and the patient’s diabetes control status[27]
are an important part of the history taking[28]
, as well as lifestyle factors such as smoking (which is considered to be the greatest modifiable factor in reducing the incidence of PAD). Other co-morbidities may provide clues to potential development of foot complications. Furthermore, poor eyesight reduces the ability to self-care and renders the individual unable to conduct daily visual checking of their own feet. Foot ulceration is more common in those with a previous history of ulceration or amputation[29]
, with rates of re-ulceration as much as 40% within 1 year, 60% at 3 years, and as high as 65% at 5 years. Indeed, it has been argued that the term ‘remission’ should be used rather than ‘healed’ when a foot ulcer is resolved[30]
. An individual with diabetes who is on dialysis (renal replacement therapy) has an increased risk of lower limb complications and are, therefore, considered high risk[31]
. Patients at increased or high risk should be referred to the Foot Protection Service.
Podiatric observation and assessment
Early intervention and treatment of any foot pathology can reduce a patient’s risk of developing a foot problem that has the potential to become ulcerative. When assessing the foot, the following should be checked:
- Are the nails thickened or discoloured?
- What condition is the skin in?
The most common condition reported on a person’s skin with diabetes are cutaneous infections (mainly fungal), followed by dry skin, which has been shown to be more prevalent with poor diabetes control[32]
.
If these conditions are observed in a patient with diabetes, it is important to refer them to an appropriate healthcare professional (e.g. podiatrist or GP) for advice/treatment to prevent simple pathologies causing tissue breakdown.
Vascular assessment
The pulses in the foot must be tested. The dorsalis pedis (DP) is found dorsally between the first and second metatarsal shafts and the tibialis posterior (PT) is found behind the medial malleolus. However, the absence of pulses alone may not indicate the presence of PAD. Further tests need to be carried out[33]
and an onwards referral should be made.
Capillary filling time following pressure applied to the skin on the apices of digits, enough to blanch and a return of normal colour, should occur within five seconds. Slower rates may indicate inadequate blood flow. No blanching in a cyanotic foot is a dangerous sign and gangrene may follow. The reliability of this test has been questioned, but it does form part of the suggested PAD examination within the NICE guidance and is widely used[34]
.
Colour of the skin (e.g. white, brick red, cyanotic); absence of hair; loss of tissue viability and elasticity; atrophy; and dry skin are all indicators of potential vascular impairment and should form part of a vascular foot assessment.
Neurological assessment
Screening for neuropathy in patients with diabetes is vital as the vast majority of neuropathic patients are asymptomatic. Various tests can be used and assessment can be divided into three categories: small, large and motor fibre tests. Small fibres are responsible for sensations such as pain and temperature perception, whereas large fibres are responsible for vibration, light touch and pressure.
The 10g monofilament is the most commonly used tool for screening of neuropathy in the foot[9]
. The monofilament should avoid areas of callus, with various combinations of test sites recommended (from 3 to 10), but no agreed consensus. However, the plantar metatarsal pad and terminal phalanges of toes are common areas for neuropathic ulceration and are the areas that are tested whether it is 3 points or 10 points that are tested.
Combining the monofilament testing with clinical examination for deformity and palpation of pulses enables the identification of patients who are 32-times more likely to ulcerate[35]
. More information can be found here[36]
.
The Ipswich touch test was devised to be able to assess for neuropathy without the use of aids (e.g. a monofilament), which could be a barrier for testing for neuropathy in some areas. Using the index finger, light touch is applied to the apex of the first, third and fifth toes on both feet. If two or more sites are not felt, the patient is considered to have neuropathy[37]
.
Risk status and referral
Following a full assessment, a patient’s risk status is determined using the NICE guidance[7]
(see table 1).
| Table 1: Assessing a patient’s risk status for developing a diabetic foot problem | |
|---|---|
| Risk status | Recommended review |
Low risk No risk factors present except callus | Annual |
Moderate risk
| 3–6 months with foot protection service |
High risk
| 1–2 months or 1–2 weeks if have a concern with foot protection service |
Active diabetic foot problem
| Refer the person within 1 working day to the multidisciplinary foot care service or foot protection service (according to local protocols and pathways; also see recommendation) for triage within 1 further working day |
Active ulceration/Charcot neuroarthropathy
Often referred to as ‘Charcot foot’, Charcot neuroarthropathy affects the bones/joints and soft tissues of the foot, which in the first stages presents as inflammation (i.e. a hot, red and swollen foot; sometimes painful; see Figure 1). It is found where there is neuropathy, of which diabetes is the most common aetiology. It is a potentially devastating condition that can lead to severe deformity of the affected foot, the most common of which is the ‘rocker-bottom type foot’[38]
, where there is a ‘disorganisation’ of bone leading to deformity as shown in Figure 1. If suspected, urgent referral within one working day to a multidisciplinary team is recommended to provide non-weight bearing treatment until a diagnosis is made[7]
.
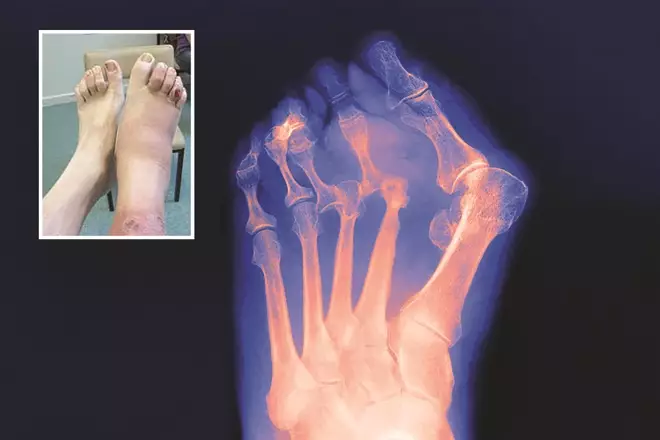
Figure 1: Charcot foot
Source: Biophoto Associates / Science Photo Library
X-ray of later stage Charcot foot, described as ‘rocker-bottom type foot’, where there is ‘disorganisation’ of the bone that results in deformity. The inset image shows the initial stages of Charcot foot, where the foot presents as red, hot and swollen.
If a foot ulcer is detected either by a healthcare professional or patient, a referral should be made within 24 hours to a multidisciplinary diabetic foot team, as shown in table 1.
Ulcers can be neuropathic (60–70%), often occurring on the apex of toes and plantar aspect of the foot; ischaemic (15–20%), which are often found on the lateral medical borders of the foot as a result of rubbing; or a combination of both, which is known as neuroischaemic (15–20%)[39]
.
NICE also recommends that healthcare professionals should be aware that if a person with diabetes fractures their foot or ankle, it may progress to Charcot arthropy[7]
.
Wound assessment and management
Different wound classifications systems are used to evaluate a wound for communication between healthcare professionals and to evaluate and improve outcomes[40]
. Both the University of Texas Wound Classification System and the SINBAD Wound Classification System are recommended by NICE[7]
(tables 2 and 3).
| Table 2: University of Texas Wound Classification System | |
|---|---|
| Grade | |
| 0 | Completely epithelialised pre-or post-ulcerative lesions |
| 1 | Superficial wounds penetrating through the epidermis only or both the epidermis and dermis |
| 2 | Wounds penetrating into tendon or capsule but not bone |
| 3 | Wounds penetrating to bone or into joint |
| Stage | |
| A | No infection or ischaemia |
| B | Infection only present |
| C | Ischaemia only present |
| D | Both infection and ischaemia present |
| Table 3: SINBAD Wound Classification System | ||
|---|---|---|
| Site | Forefoot Midfoot and hindfoot | 0 1 |
| Ischaemia | Pedal blood flow evident (one pulse present) Clinical evidence of reduced blood flow | 0 1 |
| Neuropathy | Protective sensation intact Protective sensation absent | 0 1 |
| Bacterial infection | None Present | 0 1 |
| Area | Ulcer <1cm2 Ulcer >1cm2 | 0 1 |
| Depth | Ulcer affects cutaneous tissue Ulcer reaching tendon; muscle or deeper | 0 1 |
| Total possible score | 6 | |
The classic signs of infection may not be present in a diabetic foot ulcer because of the high incidence of PAD and neuropathy. Therefore, healthcare professionals need to be more mindful of subtle signs: increase in wound exudate, friable granulation tissue, malodour and undermining of the wound[41]
.
Treatment
Offloading has been shown to significantly heal more neuropathic ulcers; the gold standard of which is a total contact cast[42]
. However, it is not advocated where there is PAD and/or infection, and alternative treatment strategies should be sought[43]
, such as a heel offloading device until revascularisation allows for a cast.
Dressings
There is no strong evidence on wound care for a diabetic foot ulcer[44]
, and NICE recommends that further good quality research is needed in this area[7]
.
Health education
Structured patient education has shown to improve diabetes control[45]
, however, there is no evidence that the same is true for foot health education. Although it is considered that it is an important part of prevention and treatment of diabetic foot ulceration[46]
. Patients should be provided with both written and oral information on basic foot care advice (including footwear), the importance of foot care, foot emergencies and who to contact, and their individual risk of developing a foot problem[7]
. Leaflets are available on various websites, such as[47]
.
A recent study identified that diabetes increases the risk for cognitive dysfunction and dementia, and people with a diabetic foot ulcer struggle even more with their ability to retain health information[48]
. This may explain non-adherence, which is important for a good clinical outcome. However, further research is needed to support this.
The role of pharmacists and other healthcare professionals
In June 2017, NHS England published the ‘RightCare pathway’ for diabetes, which includes “core components of an optimal diabetes service”. Seven key areas have been identified providing guidance for commissioners on improving outcomes for individuals with diabetes. One of the key areas identified was a reduction of major amputations (above or below the knee). It recommends that there is a triage to specialist services (30% of hospitals do not have a multidisciplinary team); a Root Cause Analysis of amputations with an outcome measure of a reduction in major amputations[49]
. The ‘National diabetes foot care audit’, published in March this year, demonstrated that delayed referral was associated with an increased risk of amputation and that those individuals who self-referred (30%) were less likely to have severe ulceration[5]
. With this in mind, it is important that pharmacists and healthcare professionals know their local diabetic foot pathways (e.g. are there any podiatry drop-in clinics or multi-disciplinary diabetic foot clinics in their area) for individuals who are at risk of developing a foot ulcer or who have an active foot ulceration. Pharmacists and healthcare professionals should also consider what local guidance is available, such as specific guidance for antibiotics prescribing for diabetic foot ulceration, especially if they are a non-medical prescriber.
Simple measures for all pharmacists and healthcare professionals who come into contact with people with diabetes include:
- Advising patients to check their feet daily;
- Advising patients to apply moisturising cream to dry areas but not between the toes;
- Advising patients to choose footwear that accommodates the shape and size of the foot to prevent rubbing or shearing;
- Advising patients never to walk barefoot (to prevent standing on anything that could traumatise or injure the foot);
- Advising patients to seek early advice as soon as a foot problem is identified (the National Diabetic Foot Care Audit demonstrates that this leads to shorter healing times and reduces the likelihood of amputation);
- Asking the patient if they have had a recent foot check as part of their annual assessment and, if they have not, advising them to have one, as well as how to perform daily foot checks themselves (see Box 2: Daily foot checks and footwear advice).
Box 2: Daily foot checks and footwear advice
- Check feet daily;
- For patients with reduced mobility or flexibility, using a mirror can be helpful to check the foot for changes. Alternatively, asking a friend or relative can be beneficial;
- Be aware of loss of sensation;
- Look for changes in the shape of the foot;
- Look for discolouration of the foot;
- Do not use corn removing plasters or blades;
- Look after toenails and ensure these are cut straight across;
- Wear shoes that fit properly;
- Examine shoes for sharp objects or stones before putting them on and replace ruffled innersole linings;
- Avoid socks, stockings or tights with wrinkles or prominent seams, or socks with holes or darned areas;
- Garters and stockings or socks with elastic tops should be avoided because they may restrict the circulation;
- Attend annual foot reviews.
Assessment, education and early appropriate intervention are key components in preventing and treating diabetic foot ulceration. As 80% of amputations are preceded by ulcers, it is hoped that appropriate early intervention can reduce this number.
RB provided financial support in the production of this content. The Pharmaceutical Journal retains full editorial control.
UK/SC/0617/0031i
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Diabetes UK. Facts and stats. Available at: https://www.diabetes.org.uk/Documents/Position%20statements/DiabetesUK_Facts_Stats_Oct16.pdf (accessed August 2017)
[2] Hex N, Bartlett C, Wright D et al. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabetic Med 2012;29:855–862. doi: 10.1111/j.1464-5491.2012.03698.x
[3] McInnes AD. Diabetic foot disease in the United Kingdom: about time to put feet first. J Foot Ankle Res 2012;5:26. doi: 10.1186/1757-1146-5-26
[4] Diabetes UK. Improving footcare for people with diabetes and saving money — an economic study in England. Available at: https://www.diabetes.org.uk/Upload/Shared%20practice/Improving%20footcare%20economic%20study%20(January%202017).pdf (accessed August 2017)
[5] NHS Digital. National Diabetic Foot Care Audit 2014–2016. Available at: http://www.content.digital.nhs.uk/catalogue/PUB23525 (accessed August 2017)
[6] Diabetes UK. Six step guide to improving diabetes footcare. Available at: https://www.diabetes.org.uk/Documents/campaigning/0997B_Putting%20feet%20first%206%20steps%204%20sider_v2_31May_web.pdf (accessed August 2017)
[7] National Institute of Health and Care Excellence (NICE). NICE guideline [NG19]. Diabetic foot problems: prevention and management. Available at: https://www.nice.org.uk/guidance/ng19 (accessed August 2017)
[8] Thiruvoipati T, Kielhorn CE & Armstrong EJ. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes.World J Diabetes. 2015;6(7):961–969. doi: 10.4239/wjd.v6.i7.961
[9] Boulton AJM, Armstrong DG, Albert SF et al. Comprehensive foot examination and risk assessment. Diabetes Care 2008;31(8):1679–1685. doi: 10.2337/dc08-9021
[10] Cotter MA & Cameron NE. The aetiopathogenesis of diabetic neuropathy: metabolic theories and vascular theories. In: Boulton AJM, ed, Diabetic Neuropathy. Lancashire: Maurius Press, 1997;97–146.
[11] Jude EB & Boulton AJM. Foot problems in diabetes mellitus. Br J Podiatry 1998;1(4):117–120.
[12] Young MJ, Boulton AJM, Macleod AF et al. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–154. doi: 10.1007/bf00400697
[13] Reiber GE, Lipsky B & Gibbons GW. The burden of diabetic foot ulcers. Am J Surgery 1998;176(suppl 2A):5S–10S. doi: 10.1016/s0002-9610(98)00181-0
[14] Edmonds ME, Blundell MP, Morris ME et al. Improved survival of the diabetic foot: the role of the specialist foot clinic. Q J Med 1986;232:763–771. doi: 10.1093/oxfordjournals.qjmed.a068033
[15] Thomson FJ, Veves A, Ashe H et al. A team approach to diabetic foot care — the Manchester experience. The Foot 1991;2:75–82. doi: 10.1016/0958-2592(91)90034-9
[16] Schaper NC, van Netten JJ, Apelgvist J et al. Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diabetes Metab Res Rev 2016;32(Suppl. 1):7–15. doi: 10.1002/dmrr.2695
[17] Boulton AJM. The pathway to ulceration: aetiopathogenesis. In: Boulton AJM, Connor H, Cavanagh PR, eds.The Foot in Diabetes. 2nd edn. Wiley & Sons, Chichester: 1994;37–48.
[18] Chadwick P et al. Diabetic peripheral neuropathic pain: the primary care perspective. 2009.
[19] Young M & Matthews C. Neuropathy screening: can we achieve our ideals? In: Diabetic Foot 1998;1(1):22–25.
[20] Rajan RS, de Gray L & George E. Painful diabetic neuropathy. Continuing Education in Anaesthesia, Critical Care & Pain 2014;14(5);230–235. doi: 10.1093/bjaceaccp/mkt063 (accessed August 2017)
[21] National Institute of Health and Care Excellence (NICE). Clinical guideline [CG173]. Neuropathic pain in adults: pharmacological management in non-specialist settings. Available at: https://www.nice.org.uk/guidance/cg173 (accessed August 2017)
[22] Bus SA, Yang QX, Wang JH et al. Intrinsic muscle atrophy and toe deformity in the diabetic neuropathic foot. A magnetic resonance imaging study. Diabetes Care 2002;25(8):1444–1450. doi: 10.2337/diacare.25.8.1444
[23] Vinik A, Maser RE, Mitchell BD et al. Diabetic autonomic neuropathy. Diabetes Care 2003;26(5);1553–1579. doi: 10.2337/diacare.26.5.1553
[24] Boulton A, Scarpello JH & Weard JD. Venous oxygenation in the diabetic neuropathic foot: evidence of arterio-venous shunting. Diabetologia 1982;22:6–8. doi: 10.1007/bf00253861
[25] Diabetes UK. State of the nation 2016: time to take control of diabetes. Available at: https://www.diabetes.org.uk/Professionals/Position-statements-reports/Statistics/State-of-the-Nation-2016-Time-to-take-control-of-diabetes/ (accessed August 2017)
[26] Kong MF & Gregory R. Preventing foot complications in diabetes: the St Vincent Declaration 26 years on. Pract Diab 2016;33:154–156a. doi:10.1002/pdi.2025
[27] Nathan DM. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: overview.Diabetes Care 2014;37(1):9–16. doi: 10.2337/dc13-2112
[28] Nickless G & Davies R. How to take an accurate and detailed medication history. The Pharmaceutical Journal 2016;296(7886). doi: 10.1211/PJ.2016.20200476
[29] Boulton AJM, Connor H, Cavanagh PR. The foot in diabetes. 1994. 2nd ed. P42.
[30] Armstrong DG, Boulton AJM & Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376(24):2367–2373. doi: 10.1056/NEJMra1615439
[31] Ndip A, Lavery LA, LaFontaine J et al. High levels of foot ulceration and amputation risk in a multiracial cohort of diabetic patients on dialysis therapy. Diabetes Care 2010;33(4):878–880. doi: 10.2337/dc09-2007
[32] Demirseren DD, Emre S, Akoglu D et al. Relationship between skin diseases and extracutaneous complications of diabetes mellitus: clinical analysis of 750 patients. Am J Dermatol 2014;15:65–70. doi: 10.1007/s40257-013-0048-2
[33] Vowden K & Vowden P. Hand-held doppler ultrasound: the assessment of lower limb arterial and venous disease. 2002. Available at: http://www.arjohuntleigh.net/diagnostics/Admin/files/20081022123919.pdf (accessed August 2017)
[34] National Institute of Health and Care Excellence (NICE). Clinical guideline [CG147]. Peripheral arterial disease: diagnosis and management. Available at: https://www.nice.org.uk/guidance/cg147 (accessed August 2017)
[35] Rith-Najarian SJ, Stolusky T & Gohdes DM. Identifying patients at high risk of lower extremity amputation in a primary health care setting: a prospective evaluation of simple screening criteria. Diabetes Care 1992;15:1386–1389. doi: 10.2337/diacare.15.10.1386
[36] North Devon Healthcare NHS Trust. Podiatry services. Screening of the diabetic foot. How to use a 10g monofilament. Available at: http://www.rdehospital.nhs.uk/docs/patients/services/diabetes/use-of-the-10g-monofilament-in-the-screening-of-the-diabetic-foot.pdf (accessed August 2017)
[37] Rayman G, Vas PR, Baker N et al. The Ipswich Touch Test. A simple and novel method to identify inpatients with diabetes at risk of foot ulceration. Diabetes Care 2011;34(7):1517–1518. doi: 10.2337/dc11-0156
[38] Rogers LC, Frykberg RG, Armstrong DG et al. The Charcot foot in diabetes. Diabetes Care 2011;34(9):2123–2129. doi: 10.2337/dc11-0844
[39] Steed DL. Foundations of good ulcer care. Am J Surg 1998;176(suppl 2A): 20S–25S. doi: 10.1016/s0002-9610(98)00178-0
[40] Ince P, Abbas ZG, Lutale JK et al. Use of the SINBAD classiï¬cation system and score in comparing outcome of foot ulcer management on three continents.Diabetes Care 2008;31(5):964–967. doi: 10.2337/dc07-2367
[41] Edmonds ME & Foster AVM. Diabetic foot ulcers. BMJ 2006;332(7538):407–410. doi: 10.1136/bmj.332.7538.407
[42] Mueller MJ, Diamond JE, Sinacore DR et al. Total contact casting in treatment of diabetic plantar ulcers: controlled clinical trial.Diabetes Care 1989;12(6):384–388. doi: 10.2337/diacare.12.6.384
[43] Nabuurs-Franssen MH, Sleegers R, Huijberts MS et al. Total contact casting of the diabetic foot in daily practice. Diabetes Care 2005;28(2):243–247. doi: 10.2337/diacare.28.2.243
[44] Game FL, Apelqvist J, Attinger C et al; and on behalf of the International Working Group on the Diabetic Foot (IWGDF). Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev 2016;32:154–168. doi: 10.1002/dmrr.2707
[45] Howorka K, Pumprla J, Wagner-Nosiska D et al. Empowering diabetes out-patients with structured education: short-term and long-term effects of functional insulin treatment on perceived control over diabetes. J Psychosom Res 2000;48(1):37–44. doi: 10.1016/s0022-3999(99)00074-4
[46] Dorresteijn JAN & Valk GD. Patient education for preventing diabetic foot ulceration. Diabetes Metab Res Rev 2012;28:101–106. doi: 10.1002/dmrr.2237
[47] Diabetes in Scotland. Publications (leaflets). Available at: http://www.diabetesinscotland.org.uk/Publications.aspx?catId=2 (accessed August 2017)
[48] Natovich R, Kushnir T, Harman-Boehm I et al. Cognitive dysfunction: part and parcel of the diabetic foot. Diabetes Care 2016;39(7):1202–1207. doi: 10.2337/dc15-2838
[49] NHS England. NHS RightCare. Diabetes pathway. Available at: h ttps://www.england.nhs.uk/rightcare/intel/cfv/pathways/diabetes-pathway/ (accessed August 2017)