Shutterstock.com
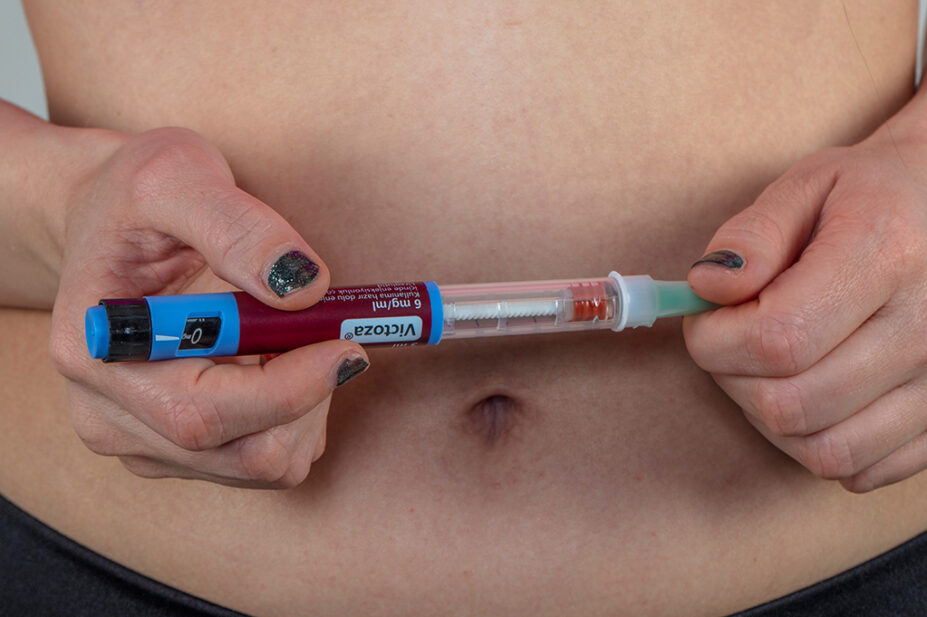
Novo Nordisk has confirmed that its Victoza (liraglutide) 6mg/mL pre-filled injection pens have been discontinued.
The pens, used in the treatment of type 2 diabetes mellitus (T2DM), have been out of stock in the UK since August 2023, having experienced supply issues along with other glucagon-like peptide-1 receptor agonists (GLP-1 RAs) since June 2023.
In a statement made to The Pharmaceutical Journal on 17 October 2024, Novo Nordisk said the product had been discontinued “as part of our broader efforts to consolidate our portfolio”.
“We are in close dialogue with the Department of Health and Social Care [DHSC], who will issue guidance, which typically includes information on the timelines, alternative treatment and next steps to be taken by healthcare professionals,” it added.
The manufacturer confirmed it had no plans to discontinue its Saxenda (liraglutide) pre-filled injection pens, which have been in restricted supply in the UK since shortages began.
Hannah Beba, consultant pharmacist for diabetes at West Yorkshire Health and Care Partnership, commented: “Victoza has been a useful drug in our journey for better care for people living with diabetes. Although I am sorry to not have it available for use anymore, we expect, over the coming years, to have a huge influx of incretin therapies — there will be no shortage of options.”
Philip Newland-Jones, consultant pharmacist for diabetes and endocrinology at University Hospital Southampton NHS Foundation Trust, said: “As there are no patients currently prescribed liraglutide on the NHS because of the previous medicines supply notifications and [it being out of stock] for the past 12 months, there should be minimal impact to patients.
“There are biosimilar liraglutide medicines already approved in the UK, which should be available shortly, and also others currently in application stage for licence in the UK, which are likely to be able to meet future market need.”
GLP-1 RAs are used for managing blood glucose levels in people with T2DM, and for weight loss, but have experienced a surge in the off-label use of diabetes products in weight loss over the past two years.
In June 2023, the DHSC issued a medicine supply notification that said a “high impact” shortage of GLP-1 RAs had been caused by “an increase in demand for these products for licensed and off-label indications”.
In January 2024, the DHSC warned shortages of GLP-1 RAs were likely to continue for the rest of the year.