Shutterstock.com
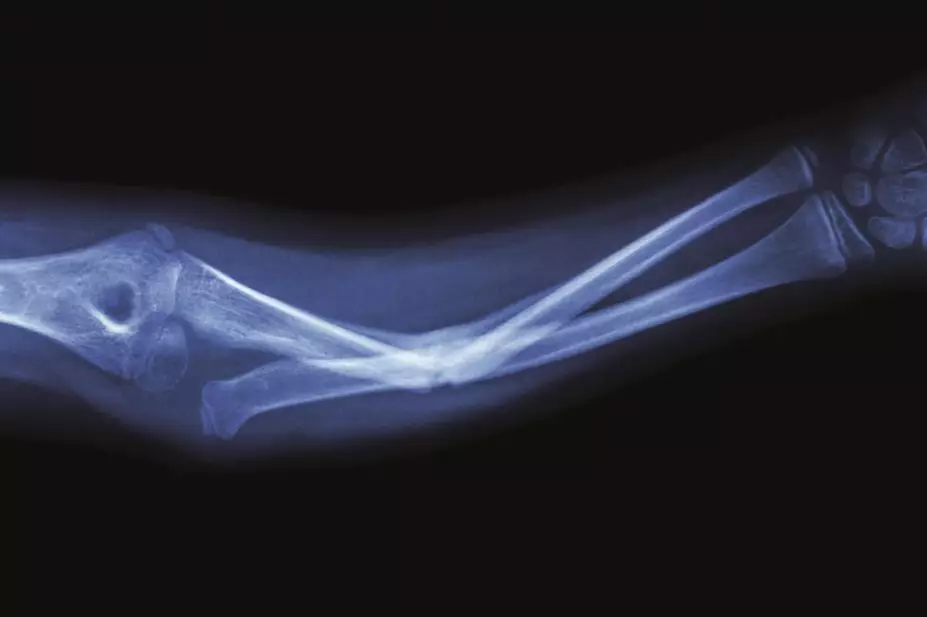
The US medicines safety watchdog has strengthened its warnings about the risk of bone fractures for type 2 diabetes patients who take canagliflozin.
The Food and Drug Administration (FDA) says it has fresh evidence that suggests fractures can occur after 12 weeks of taking the drug, which in the United States is branded by Janssen as Invokana and Invokamet (in the UK, Invokamet is known as Vokanamet).
The FDA is also warning patients and health professionals that the drug — a sodium-glucose cotransporter-2 (SGLT2) inhibitor — has the potential to decrease bone mineral density in the hips and lower spine.
The agency has revised the drug label and added a new warning and precaution to reflect its new concerns about possible fracture, the FDA announced on 10 September 2015.
The move by the FDA follows new evidence from clinical trials.
A spokesperson for the European Medicines Agency (EMA) says it has already assessed these risks and that the EU product information for canagliflozin lists bone fractures as a side effect.
“Based on an assessment of the same data, the EMA concluded that the observed changes in bone density were very small and were unlikely the cause of the observed fractures,” the spokesperson says, adding that the EMA will continue to monitor the effects of SGLT2 inhibitors, and “will take any necessary measure to ensure that these medicines are used as safely as possible”.