MAG / Shutterstock.com / Science Photo Library
Imagine suffering from blurred vision, regular headaches, numbness and tingling in your arms and legs, constantly feeling tired and struggling to walk or even get dressed alone. This is you at your best. Before long, you might experience incontinence, be unable to think clearly and will perhaps need a wheelchair to get around. As the years go by, you may even become bedbound, increasing your risk of death from potentially life-threatening complications, such as pneumonia, that can result from reduced mobility.
The devastating reality for many people suffering from progressive multiple sclerosis (PMS), an unrelenting form of the disease, is that there are no effective treatments to halt its advance. This is in stark contrast to the relapsing form of the illness, which is characterised by periods of partial or complete remission, and for which several disease-modifying drugs are available.
A lack of understanding of the underlying biology of PMS has made it difficult to identify targets and design effective trials, says Timothy Coetzee, chief research officer of the National MS Society in the United States.
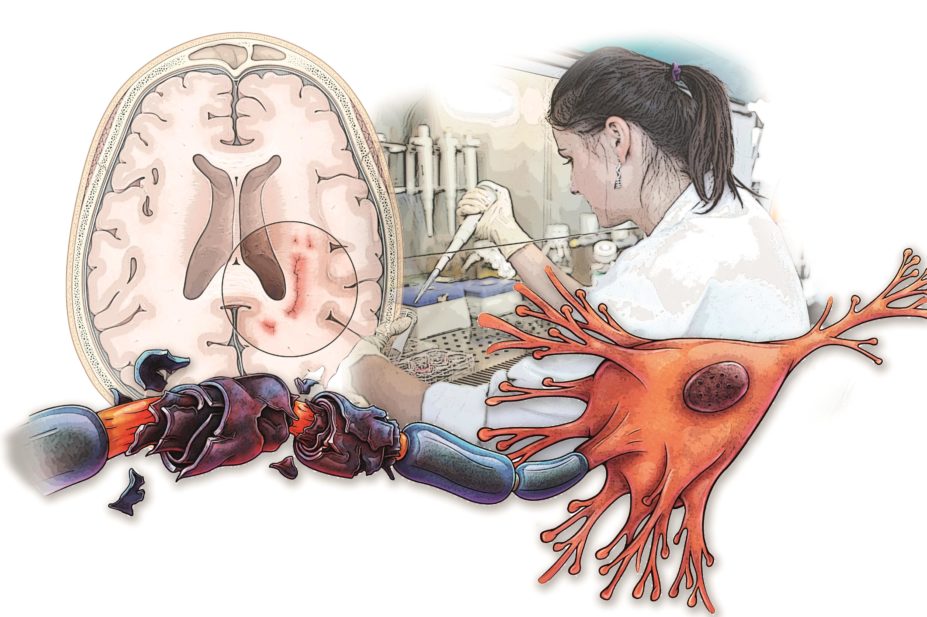
Multiple sclerosis (MS) is a chronic disease of the brain and spinal cord that occurs when myelin, the protective layer of fatty substance that encases nerve fibres — like the plastic coating that insulates electrical wire — comes under attack from the immune system. Lesions form, slowing and disrupting the electrical signals and causing a variety of symptoms depending on which part of the nervous system is damaged. Disability increases over the subsequent decades as the nerve fibres themselves become injured or even die.
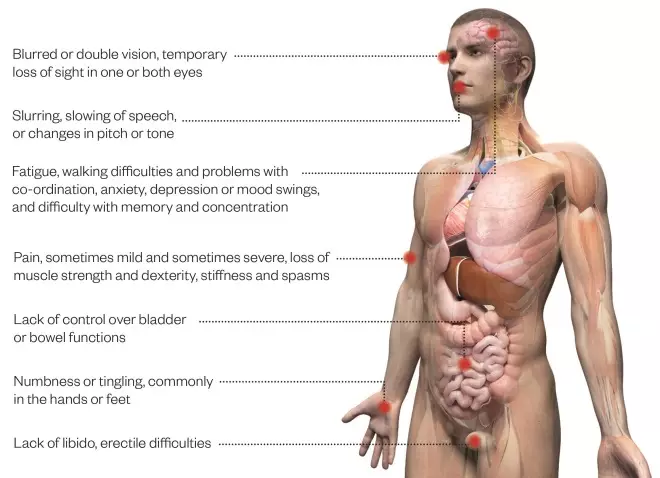
Common symptoms of multiple sclerosis
Source: MS Society
Progressive multiple sclerosis shares many of the same symptoms as relapsing remitting multiple sclerosis but symptoms gradually worsen over time. Disease progression varies from person to person, and over time, and there can be long periods where there is no noticeable change.
Around 33 people per 100,000 worldwide have MS. It is most prevalent in North America and Europe, where the number increases to more than 100 people per 100,000.
About 15% of people are initially diagnosed with PMS — so-called primary PMS (PPMS). The remaining 85% are diagnosed with relapsing remitting MS (RRMS), but within 15 years about 65% of them will develop PMS, so-called secondary PMS (SPMS). PPMS is usually diagnosed when people are in their 40s or 50s — older than the average age for RRMS — and equal numbers of men and women are affected.
Much progress has been made over the past 20 years in developing disease-modifying treatments for RRMS. These can reduce the number of relapses by between 30% and 70% and may lessen the rate of disability over time. Eleven such drugs are currently approved for use on the NHS to treat RRMS, but all of them have failed in tests to treat PMS.

Courtesy of Robert Fox
Robert Fox, vice-chair for research, Neurological Institute, Cleveland Clinic, Ohio, believes that the pathophysiology of progressive multiple sclerosis is very different from relapsing multiple sclerosis
“We presumed progressive MS had the same pathophysiology as relapsing MS, so applied all the same therapies,” says Robert Fox, vice-chair for research, Neurological Institute, Cleveland Clinic, Ohio. “I believe the pathophysiology of progressive MS is very different from relapsing MS — hence the same therapies yield disappointing results.”
Unlike RRMS, there is no animal model or effective clinical outcome measures for progressive disease. Nor are there validated imaging tools and biomarkers to monitor the disease and assess the effect of a drug on the brain over a relatively short time before clinical symptoms become apparent. But change may be on the horizon, with recent advances in understanding the complex pathogenesis of PMS, as well as efforts to improve outcome measures, identify biomarkers and refine clinical trial design.
Duality
Damage to myelin and nerve cells is the hallmark of MS, but the definitive cause is still unknown; the origin of the autoimmune response and neurodegeneration remains unclear.
It is generally thought that in the initial stages of MS (leading to the relapsing-remitting form), environmental, lifestyle or infectious triggers cause T lymphocytes and B lymphocytes to infiltrate the brain, resulting in inflammation. This in turn attracts macrophages and activates local immune cells, such as microglia and astrocytes, which are involved in processes, including producing cytokines and chemokines, that damage myelin and myelin-producing oligodendrocytes. This leads to the demyelinated plaques and neurodegeneration responsible for clinical disease. At this stage, blocking the T and B cells involved in the initial inflammation can prevent new lesions forming. This is how most of the RRMS therapies work.
Progressive disease, however, is slightly different. Current thinking is that inflammation caused by T cells and B cells diminishes, but local inflammatory processes in the central nervous system — from activated innate immune cells — continue to fuel degeneration, resulting in more diffuse brain damage (cortex, and grey and white matter). The balance is further tilted towards neurodegeneration because oxidative injury — originally driven by microglia, which produce oxygen radicals that directly damage cells — becomes amplified by mitochondrial damage and iron released in the ageing brain when myelin and oligodendrocytes are destroyed, according to Hans Lassmann, head of the Center for Brain Research at the Medical University of Vienna.
Importantly, persistent inflammation and inflammation-induced tissue injury may block neuroprotection mechanisms and remyelination, says Lassmann. Remyelinated areas become demyelinated again, and remyelination fails as myelin-producing cells are destroyed — explaining the lack of remission.
Drug development
The complex pathogenesis offers many potential targets for drug development, and Novartis, Biogen, Roche, Genentech and Opexa Therapeutics are among those hoping their approach will yield an effective therapy (see tables). Some aim to prevent accumulation of inflammation in the brain, for example, by preventing B cells from travelling there. Others aim to block the mechanisms that result in neurodegeneration, such as oxygen radical formation and iron imbalances, or repair the damage, perhaps by protecting oligodendrocytes and mitochondria or via stem cell transplants.
The only PMS drug so far to demonstrate significant efficacy in a phase III trial is Genentech’s ocrelizumab, a humanised monoclonal antibody that binds CD20-positive B cells, leaving other antibody-producing B cells to continue fighting infection. Top line results from a trial in PPMS (ORATORIO), released in September 2015[1]
, show ocrelizumab significantly reduced the progression of clinical disability for at least 12 weeks by 24% compared with placebo. It also reduced relapses and disease progression over two years in RRMS compared with interferon (OPERA)[1]
. In February 2016, the Food and Drug Administration (FDA), which is responsible for evaluating the safety and efficacy of medicines in the United States, granted breakthrough therapy designation to expedite ocrelizumab’s development and review.
For a disease where there are no other treatment options, this is a very welcome result
“For a disease where there are no other treatment options, this is a very welcome result,” says Daniel Chancellor, lead analyst at London-based Datamonitor Healthcare. “Ocrelizumab is absolutely the most promising drug in development for primary PMS.”
Chancellor assumes that ocrelizumab’s broader effect on reducing disability progression in both PPMS and RRMS will mean that it is also effective for SPMS — a role he expects the drug will fill off-label, unless Roche (Genentech’s parent company) confirms this in a study.
A previous trial of Genentech’s rituximab, an anti-CD20 mouse antibody, failed in PMS trials, but a breakthrough discovery in 2015 that a specific type of B cell appears to be a major driver of MS seems to support the rationale for targeting B cell subsets in drug development[2]
.
The immune system, however, is serpentine. Most groups of cells can both help and harm — macrophages, for example, have pro-inflammatory and anti-inflammatory roles; microglia can be both protective and damaging. Couple this with the intricate nervous system and it makes successfully modulating the right targets extremely difficult.
The good news is that much research is underway to understand the processes driving PMS. Some researchers, for example, are focusing on how oligodendrocyte death triggers attack on myelin[3]
while others are investigating the role of activated microglia in the brain[4]
. How abnormalities in mitochondrial DNA in neurons may contribute to degeneration[5]
and lead to symptoms such as fatigue is also being investigated, as is how toxic ceramides, fatty substances derived from myelin, may be involved[6]
. Other researchers are exploring the use of autologous haematopoietic stem cell (bone marrow) transplants to reset the immune system[7]
.
Neuroprotective therapies will only be effective when the chronic inflammatory process is under control
With two systems involved, however, it is likely that a combination of anti-inflammatory and neuroprotective approaches will be required for a successful treatment. “Neuroprotective therapies will only be effective when the chronic inflammatory process is under control,” says Lassmann.
Outcome measures
The effects of new therapies can only be accurately assessed with measuring tools that reliably reflect the course of the disease. Disease modifying therapies for RRMS are approved because they reduce the number of relapses over a two-year period. Relapses are well defined and can be measured, says Coetzee.
In contrast, PMS can manifest in different ways, with the disease pattern varying from person to person, making it difficult to define and measure. Since clinical progression happens slowly, typical trials are not long enough to pick up the changes caused by a new drug.
“The clinical outcome assessment tool commonly used (Kurtzke Expanded Disability Status Scale [EDSS]) is not very sensitive, meaning that the score doesn’t change for most [PMS] patients during a two-year period, which is a typical length of time for a clinical trial,” explains Lynn Hudson, chief science officer at C-Path, a non-profit organisation based in Tucson, Arizona, that aims to advance science through cross-disciplinary collaboration. “There are many reasons for this, but the most important reason is that EDSS in progressive MS patients is mostly a walking scale, and the score doesn’t reflect changes in arm or hand dexterity, vision, or cognitive function.”

Courstey of Lynn Hudson
Lynn Hudson, chief science officer at C-Path, a non-profit organisation, says typical clinical trials are not long enough to pick up the changes caused by a new drug in progressive multiple sclerosis
There is currently no sensitive surrogate that can track changes and treatment effects over relatively short intervals. Magnetic resonance imaging (MRI) measurement of the effect of treatment on new inflammatory lesions is used in RRMS because it is much more sensitive to disease activity than clinical assessment of relapses. “Treatment effect on lesions is not necessarily associated with treatment effect on clinical progression in PMS,” points out Douglas Arnold, a McGill University neurologist, based in Montreal, Canada, who specialises in MRI.
From a therapy development point of view, one critical barrier is there is no ready proof-of-concept biomarker for phase II trials
“From a therapy development point of view, one critical barrier is there is no ready proof-of-concept biomarker for phase II trials,” says Coetzee. In RRMS, gadolinium, which can pass through the blood brain barrier during a relapse and enter MS lesions (gadolinium-enhancing lesions), is used to demonstrate efficacy of a treatment during phase II trials, but there is no such tool for PMS, he says.
This could change by the end of 2016, when interim results are expected from the phase IIb study (SPRINT-MS) of MediciNova’s ibudilast, a small molecule that inhibits macrophage migration. As well as testing ibudilast versus placebo in primary and secondary PMS, the SPRINT-MS trial is directly comparing five imaging metrics in all participants to identify which is the most robust, responsive, reproducible and clinically relevant biomarker of disease progression. The measures are whole brain shrinkage, cortical atrophy, changes detected by magnetisation transfer ratio imaging and diffusion tensor imaging, and thinning of the retinal nerve fibre layer as detected by optical coherence tomography.
“The SPRINT-MS trial will help us understand if ibudilast does have bioactivity in progressive MS. Just as importantly, SPRINT-MS will help us learn how to better conduct phase II trials in progressive MS,” says Fox, who is also a neurologist at the Mellen Center for MS at Cleveland Clinic, Ohio, which is leading the trial. “In other words, it may catch a fish, but it will also teach us how to fish better.”
Many other efforts, meanwhile, are under way to develop improved or meaningful outcome measures and to refine trial design. Some researchers are investigating spinal cord imaging[8]
, while others are developing novel molecular imaging probes for use with PET scans[9]
. Researchers are also trying to design more efficient trials that enable the testing of multiple treatments on fewer people in a shorter time[10]
.
As with research to understand PMS, several of the projects are funded by the International Progressive MS Alliance, a network of MS societies established in 2012 to speed up drug development of treatments via funding and collaboration.
“We are now for PMS where we were 30 years ago for RRMS in terms of understanding, measures and trials,” says Coetzee. “The whole community is highly engaged in this and significant progress is being made — there is clear momentum and trajectory.”
| Drug | Company | Stage | Mechanism |
|---|---|---|---|
| Source: MS Trust, MS Society, MS Discovery Forum, National MS Society | |||
| Laquinimod (Nerventra) | Teva/Active Biotech | II (AREGGIO) | Tablet thought to prevent T cells entering the central nervous system and affect cytokine levels |
| Fingolimod (Gilenya) | Novartis | III (already approved for relapsing remitting multiple sclerosis) | Tablet binds to four forms of sphingosine-1-phosphate receptor 1, located on the surface of lymphocytes, trapping the lymphocytes in the lymph nodes and preventing them from reaching the CNS |
| Masitinib | AB Science | III | Tablet targets tyrosine kinase and inhibits mast cells |
| Ocrelizumab | Genentech (Roche) | III (ORATORIA) (FDA breakthrough therapy) | Antibody targets CD20-positive B cells |
| Ibudilast (MN-166) | MediciNova | IIb (SPRINT-MS) (FDA fast track) | Tablet targets phosphodiesterase and inhibits macrophage migration |
| Drug | Company | Stage | Mechanism |
|---|---|---|---|
| Source: MS Trust, MS Society, MS Discovery Forum, National MS Society | |||
| Anti-LINGO-1 | Biogen | II (SYNERGY) | Antibody blocks protein found in nerve cells and myelin-making oligodendrocytes, resulting in myelin repair |
| Masitinib (AB1010) | AB Science | III | Tablet targets tyrosine kinase and mast cells |
| MD1003 (high concentration Biotin) | MedDay | III | Capsule thought to activate a carboxylase involved in myelin synthesis |
| Natalizumab (Tysabri) | Biogen | III (already approved for relapsing remitting multiple sclerosis) | Antibody binds alpha-4 integrin (cell adhesion molecule) preventing lymphocytes from crossing the blood-brain barrier |
| Siponimod (BAF312) | Novartis | III | Tablet selectively binds to two forms of the sphingosine-1-phosphate receptor, located on the surface of lymphocytes, trapping the lymphocytes in the lymph nodes and preventing them from reaching the central nervous system |
| Tcelna (imilecleucel-T) | Opexa Therapeutics | IIb (FDA fast track) | A personalised T-cell immunotherapy that reduces the number and activity of myelin-reactive T cells |
| Ibudilast (MN-166) | MediciNova | IIb (SPRINT-MS) (FDA fast track) | Tablet targets phosphodiesterase and inhibits macrophage migration |
| Simvastatin | Repurposed (no pharma involvement) | II | Tablet thought to have neuroprotective effect via vascular system at high doses |
| Tecfidera (dimethyl fumarate) | Biogen | III (INSPIRE) | Tablet thought to decrease oxidative damage |
| Autologous haematopoietic stem cell transplantation | II (ACTiMUS) | Bone marrow transplant thought to reset the immune system | |
| Amiloride | Repurposed | II (MS-SMART) | Tablet blocks sodium channels |
| Riluzole | Repurposed | II (MS-SMART) | Tablet blocks glutamate receptors |
| Fluoxetine | Repurposed | II (MS-SMART) | Tablet selectively inhibits reuptake of serotonin |
References
[1] Roche. Roche’s ocrelizumab first investigational medicine to show efficacy in people with primary progressive multiple sclerosis in large Phase III study. Press release. 28 September 2015. Available at: http://www.roche.com/media/store/releases/med-cor-2015-09-28b.htm (accessed 28 April 2016)
[2] Li R, Rezk A, Miyazaki Y et al. Proinflammatory GM-CSF–producing B cells in multiple sclerosis and B cell depletion therapy. Science Translational Medicine 2015;7:310ra166. doi: 10.1126/scitranslmed.aab4176
[3] Traka M, Podojil JR, McCarthy DP et al. Oligodendrocyte death results in immune-mediated CNS demyelination. Nature Neuroscience 2016;19:65–74. doi: 10.1038/nn.4193
[4] Goldmann T, Wieghofer P, Müller PF et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nature Neuroscience 2013;16:1618–1626. doi: 10.1038/nn.3531
[5] Campbell GR, Worrall JT & Mahad DJ. The central role of mitochondria in axonal degeneration in multiple sclerosis. Multiple Sclerosis Journal 2014;20:1806–1813. doi: 10.1177/1352458514544537
[6] Vidaurre OG, Haines JD, Sand IK et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 2013;137:2271–2286. doi: 10.1093/brain/awu139
[7] Radaelli M, Merlini A, Greco R et al. Autologous bone marrow transplantation for the treatment of multiple sclerosis. Current Neurology and Neuroscience Reports 2014;14:478. doi: 10.1007/s11910-014-0478-0
[8] Wheeler-Kingshott CA, Stroman PW, Schwab JM et al. The current state-of-the-art of spinal cord imaging: applications. Neuroimaging 2014;84:1082–1093. doi: 10.1016/j.neuroimage.2013.07.014
[9] Institut National de la Santé et de la Recherche. PROJECT 9: Novel molecular imaging probes to predict disability progression and evaluate therapies in MS. Available at: https://scleroseforeningen.dk/project-9-novel-molecular-imaging-probes-predict-disability-progression-and-evaluate-therapies-ms (accessed 28 April 2016)
[10] Ontaneda D, Fox RJ & Chataway J. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. The Lancet Neurology 2015;14:208–223. doi: 10.1016/S1474-4422(14)70264-9
You may also be interested in
Antiepileptic drug treatment in children

Epileptic seizure: urgent action
