Science Photo Library
Patients come to Jonathan Barker’s clinic in London when they have run out of other options. Usually it’s because their psoriasis has not responded to standard treatments, or because they have other conditions that preclude the use of these drugs, or sometimes a combination of the two. So finding the best therapies to ease their medical and psychological burdens is not easy. “It can be very complicated,” says Barker, a consultant dermatologist and researcher at King’s College London, who runs a tertiary referral service for patients with severe psoriasis. “Patients are desperate for help.”
Thanks to recent advances in understanding the biological basis of psoriasis, however, clinicians such as Barker now have increasingly effective drugs at their disposal. New biologic therapies that target the immunological faults underlying the disease have come through clinical trials with encouraging results. “With approved drugs we already have the ability to treat psoriasis very well, and probably better than just about any autoimmune disease,” says James Krueger, who studies inflammatory skin diseases at Rockefeller University in New York. The new drugs, however, show even greater degrees of improvement.
Hopefully, we will come up with a better way of classifying psoriasis and understand the key pathways that should be targeted
Small-molecule drugs that block errant cell signalling pathways are also being developed and could form the basis of much-needed new topical treatments. But translating these treatments into clinical practice means striking a difficult balance between a patient’s clinical needs and a drug’s efficacy, cost and safety. So UK researchers are pioneering a unique study to find accurate, cost-effective ways of matching psoriasis patients with the treatments that are most likely to help them, and in the process are shedding new light on the molecular pathologies underlying psoriasis. “Hopefully, we will come up with a better way of classifying psoriasis and understand the key pathways that should be targeted,” says Chris Griffiths, a consultant dermatologist at the University of Manchester, UK, who is leading the study. Researchers believe this kind of study into the molecular immunology of psoriasis will be a fertile testing ground for therapeutic approaches to treat other autoimmune conditions too.
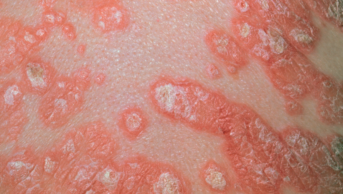
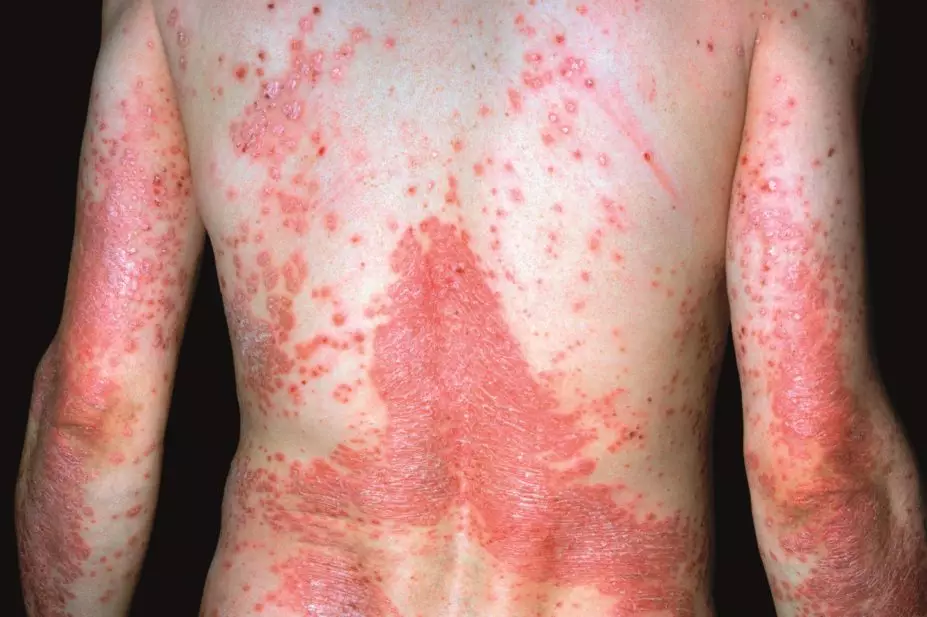
Psoriasis is a common skin condition that affects about 2% of the population in Europe and North America, and there are several forms. The most common, affecting 90% of patients, is plaque psoriasis, which results in raised, inflamed, scaly patches on the skin, scalp and nails. Psoriasis is graded by severity: ‘mild’ psoriasis involves less than 3% of the body, ‘moderate’ affects 3–10%, and ‘severe’ involves more than 10%. About 80% of patients have mild psoriasis, but ‘mild’ is something of a misnomer in terms of its impact on people’s lives, says Barker. “In great part, people feel disfigured by it.” This is particularly true for patients whose lesions are on the face or other visible parts of the body, such as their hands. In the way it impairs a person’s quality of life, psoriasis is comparable to other major medical conditions such as cancer and depression. But the need to balance the risk of side effects against therapeutic benefit — long-term use of conventional systemic treatment methotrexate can result in liver toxicity for example — has limited the treatment of mild psoriasis to topical therapies, such as corticosteroid creams. For moderate and severe cases, topical treatment is impractical, and the benefits of systemic therapies are generally considered to outweigh the risks.
Drug targets
The original idea behind drugs such as methotrexate was to restrict the excess cell division seen in skin cells called keratinocytes, because at the time this was thought to drive the disease. This notion began to change in the mid-1990s, however, when researchers realised that psoriasis is primarily an autoimmune disease. So clinicians started to explore the use of antibody-based biologics that block an immune signalling molecule, or cytokine, known as tumour necrosis factor (TNF). Several of these biologics had already been developed to treat other inflammatory conditions, such as rheumatoid arthritis, and some, including etanercept, were soon adopted for psoriasis treatment, with considerable success. “Our practice has changed enormously since their introduction,” says Barker. But these biologics increased patients’ risk of infections, probably because of their relatively broad immunosuppressive effects.
As research progressed, it became clear that psoriasis is driven by immune cells called T cells, which play a vital role in coordinating and effecting the immune response. There are various types, or subsets, of T cells, each of which performs a particular function. Early attempts to target specific subsets of T cells resulted in the development of alefacept (marketed as Amevive by Astellas Pharma US) and efalizumab (Raptiva; Genentech). Both drugs were approved by the US Food and Drug Administration (FDA) but were later discontinued. Efalizumab was withdrawn in 2009 when it emerged that long-term use was associated with strong immunosuppression and an increased risk of developing a rare and serious viral illness called progressive multifocal leukoencephalopathy (PML). Alefacept was discontinued in 2011, a decision Astellas said was driven by business needs.
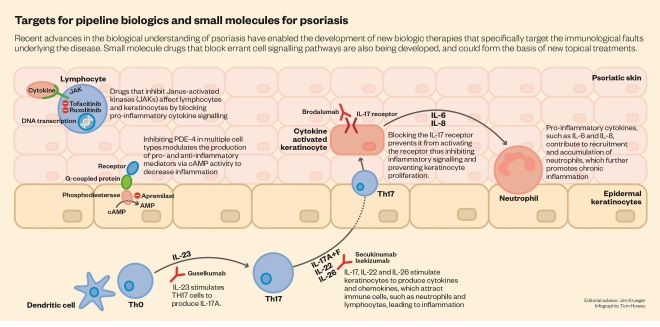
Meanwhile, immunologists were uncovering more detail about the roles of the different subsets of T cells and the cytokines they use to communicate with the rest of the immune system. Helper T cells, which regulate the behaviour of other immune cells, were initially divided into two subsets: TH1 and TH2. Researchers identified TH1 cells as being central to driving the inflammatory response in psoriasis, so they tried to find cytokines that could specifically affect their behaviour. This led them to focus on interleukin-12 (IL-12), a cytokine that promotes TH1 cell development.
Ustekinumab (Stelara; Centocor/Janssen), a monoclonal antibody that targets a subunit of IL-12, was approved to treat moderate and severe psoriasis in 2009. It led to an improvement of at least 75% in a standard measure of psoriasis severity, known as the psoriasis area and severity index (PASI 75), in about 70% of patients[1]
. A later trial showed that it was more effective than etanercept, which resulted in 56% of patients achieving PASI 75[2]
.
In a surprising twist, however, it emerged that ustekinumab’s success was achieved more by accident than design. The IL-12 subunit targeted by the antibody turned out to be shared by another cytokine, called IL-23, which regulates another subset of T cells, known as TH17. It became clear that TH17 cells, which produce a further cytokine, IL-17, play an important role in several autoimmune conditions, including psoriasis. And because TH17 cells normally control only a small part of the immune response (against mucosal fungal infections), drugs directed against them are expected to have fewer side effects. “The cells that make IL-17 comprise normally only 2% or 3% of T lymphocytes. So that leaves behind more than 90% of lymphocytes, which are not being interfered with and presumably mediate other types of protective immune functions,” says Krueger. “So, at least on theoretical grounds, the least havoc that could be had to protective immunity would occur with blocking IL-17.”
Further investigation revealed that psoriasis patients produce abnormally high levels of IL-23. This causes their TH17 cells to make IL-17, also known as IL-17A, which helps to attract other immune cells that boost the activity of keratinocytes. Researchers then developed antibodies that target IL-17A (secukinumab and ixekizumab) and its receptor (brodalumab), along with antibodies that target IL-23 alone (guselkumab). Results from clinical trials have demonstrated that this approach is remarkably effective as well as confirming the important role of TH17 cells in psoriasis.
The first of the biologics targeted at IL-23 and IL-17A to gain regulatory approval was secukinumab, marketed as Cosentyx by manufacturer Novartis. In 2014, phase III trials demonstrated excellent efficacy and safety profiles, and better efficacy than etanercept[3]
. And in June 2015, a study found that secukinumab’s ability to induce a 90% improvement (PASI 90) in skin symptoms was superior to that of ustekinumab[4]
.
Next on the market was Eli Lilly’s ixekizumab. The results of two phase III trials were published in June 2015 and showed that it too is more effective than etanercept[5]
. The company says it will submit the antibody to regulatory bodies in 2015. Johnson & Johnson’s guselkumab is currently in phase III trials. Brodalumab was also showing promise in phase III trials when its manufacturers, Amgen and AstraZeneca, announced in May 2015 that they would cease developing the drug after some users reported having suicidal thoughts.
Money matters
Clinicians and policymakers need to balance the risk of long-term side effects against the clear benefits of the new biologics. The safety data are generally reassuring, but the drugs are still new, and trial data are available for only up to a year or two of use. “Biologics are of huge importance to patients with severe disease,” says Barker, “but we just need to temper our enthusiasm with the fact that they are new and they are expensive.”
We just need to temper our enthusiasm with the fact that they are new and they are expensive
A year’s course of biologic psoriasis treatment costs about £10,000 per patient, making it a thousand times more expensive than a similar course of methotrexate (just £10 per patient per year). Although there is some wiggle room on price — Novartis has agreed to offer Cosentyx at a discount to the NHS, for example — the cost makes it unlikely that biologics will soon be first-line treatments. Cosentyx, for example, is only approved for restricted use within the NHS in Scotland, England and Wales.
Related to cost-effectiveness is the issue of how individual patients respond to biologic therapy: not all of them experience significant improvement. Even those who do usually find that efficacy decreases over time, with different biologics waning at different rates. “Currently we prescribe drugs in a somewhat trial-and-error manner,” says Griffiths. “We can’t just by looking at a patient know which drug he or she is going to respond best to, with minimal side effects, without a lot more information.”
Getting personal
Griffiths and his team at Manchester are now heading a consortium of researchers — including Barker and his colleagues at King’s College London — and industry partners that aims to get this information. The Psoriasis Stratification to Optimise Relevant Therapy (PSORT) programme, funded with £5m from the UK Medical Research Council and £2m from the pharmaceutical industry, plans to undertake detailed studies of the genetics, transcriptomics, immunology, pharmacology, clinical data and treatment adherence of patients with chronic plaque psoriasis who receive biologic therapies, and correlate these with treatment outcomes. Essential to the project is the Manchester-based British Association of Dermatologists’ Biologic Interventions Register, a long-term safety register of patients on biologic therapies for psoriasis, which was set up in 2007 and now involves more than 10,000 patients and 150 dermatology centres across the UK and Ireland.
What we hope to do is come up with a new taxonomy for psoriasis
As is already happening in several other diseases, such as cancer, the aim of PSORT is to stratify plaque psoriasis patients into groups defined by their underlying molecular pathologies, rather than their outward clinical symptoms. “It’s not a single disease — it’s probably a number of diseases that look very similar,” says Griffiths. “What we hope to do is come up with a new taxonomy for psoriasis.” As well as allowing doctors to tailor treatments more effectively, stratifying patients into more homogeneous groups should aid the development of new therapies, he adds. Launched in September 2014, the study is currently deep into its recruitment phase and hopes to see the first results emerge in 2016.
Although PSORT’s scope and comprehensive approach make it unique, the basic idea of undertaking detailed molecular studies to better understand patients’ response to therapy and uncover new insights into disease mechanisms is being pursued by psoriasis researchers around the world. “The ability to do this, to unsort the molecular pathways, and to use that to improve care is an evolving set of findings and one that we all view as very important,” says Krueger. “A lot of it is in the realm of personalised medicine, maximising responses and making clinical trials for discovering new agents as efficient as possible.” Krueger and his colleagues, for example, have described a set of genes that are differentially active in normal and psoriatic skin, and are studying how gene expression alters when patients receive treatment[6]
.
Small molecules
Molecular studies are also allowing researchers to uncover more about the immune signalling mechanisms that go awry in psoriasis, and these offer new targets for intervention with small-molecule drugs. Evidence that this is a potentially fruitful approach comes from the small molecules already in use or in the later stages of development. Apremilast (Otezla; Celgene), for example, is a phosphodiesterase inhibitor that interferes with intracellular signalling and reduces the production of cytokines that promote inflammation. Results from a phase III trial reported in July 2015 showed that apremilast is well tolerated and has induced PASI 75 in about 60% of patients with severe or moderate plaque psoriasis[7]
. The FDA approved its use in psoriatic arthritis and plaque psoriasis in March and September 2014, respectively, with EU approval following in January 2015. In the UK, it is currently approved for patients with psoriatic arthritis who meet certain conditions.
Another promising small-molecule oral therapy is tofacitinib, which blocks intracellular signalling brought about by enzymes known as Janus kinases (JAKs). Tofacitinib is already used to treat rheumatoid arthritis, and has shown positive results in clinical trials for psoriasis, so its manufacturer, Pfizer, has submitted it for FDA approval for use in psoriasis.
There’s been no advance in topical medicines since the early 1990s
Small molecules are generally less effective than biologics and can be just as expensive — a year’s course of apremilast costs about £7,000, for example. But they do offer some advantages, says Barker, such as not requiring the regular blood tests that biologics demand. “It’s very easy for practitioners to prescribe,” he says. Another potential advantage is that, unlike biologics, small molecules are able to penetrate the skin and so could form the basis of new topical therapies, which would be particularly welcome for patients with mild psoriasis. Tofacitinib ointment is currently undergoing trials, as is another JAK inhibitor, ruxolitinib (Jakavi; Incyte/Novartis), which is currently used to treat certain bone-marrow disorders. “I think there is a need for better topicals,” says Barker. “There’s been no advance in topical medicines since the early 1990s.”
Some researchers have made a case for treating patients who have mild psoriasis with systemic therapies. Although it manifests in the skin, psoriasis is a systemic autoimmune disease, and many researchers think that its comorbidities, such as metabolic syndrome and cardiovascular disease, may be attributable to the generalised inflammation seen in psoriasis. But this association has yet to be conclusively demonstrated, and potential side effects, drug licensing and cost are likely to preclude systemic treatment, at least for the time being.
Nevertheless, the easy accessibility of the skin, and the fact that patients are usually treated with only one drug at a time, means that psoriasis research is paving the way for improved treatments for other autoimmune diseases that are harder to study. “There are improvements happening across inflammation, and I’m really happy that what we’ve done in psoriasis has been as successful as it is,” says Krueger. “I hope it may serve as a model for trying to approach some of these other diseases in other tissues.”
References
[1] Leornardi CL, Kimball AB, Papp KA et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371:1665–1674. doi:10.1016/S0140-6736(08)60725-4
[2] Griffiths CEM, Strober BE, van de Kerkhof P et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. New England Journal of Medicine 2010; 362:118–128. doi:10.1056/NEJMoa0810652
[3] Langley RG, Elewski BE, Lebwohl M et al. Secukinumab in plaque psoriasis — results of two phase 3 trials. New England Journal of Medicine 2014;371:326–338. doi:10.1056/NEJMoa1314258
[4] Thaçi D, Blauvelt A, Reich K et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. Journal of the American Academy of Dermatology 2015;73:400–409. doi:10.1016/j.jaad.2015.05.013
[5] Griffiths CEM, Reich K, Lebwohl M et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet 2015;386:541–551. doi:10.1016/S0140-6736(15)60125-8
[6] Tian S, Krueger JG, Li K et al. Meta-analysis derived (MAD) transcriptome of psoriasis defines the “core” pathogenesis of disease. PLoS One 2012;7:e44274. doi:10.1371/journal.pone.0044274
[7] Papp K, Reich K, Leonardi CL et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). Journal of the American Academy of Dermatology 2015;73:37–49. doi:10.1016/j.jaad.2015.03.049